Dear Readers,
If you are new to SciX newsletter and want to get a view of what to expect in the articles, please click here.
In this article, we will take a deep dive into CAR-NK cell therapy landscape. We will discuss the 1) unique properties of NK cells over T cells, 2) explore multiple biotech companies and academic institutions developing CAR-NK cell therapy products (Nkarta, Fate Therapeutics, Cytoimmune, Glycostem, Immunity Bio, Catamaran Bio, Wugen, MDACC, Takeda, Artiva Biotherapeutics, PearsonGen BioTherapeutics, Athenex (Kuur Therapeutics), Sanofi (Kiadis), and Affimed), and compare their CAR-NK assets, 3) learn about the ongoing clinical trials and interim findings and finally 4) discuss the limitations of CAR-NK cell therapy.
Hopefully by the end of this long post you will understand the science behind the design of CAR-NK cells and the future of NK cells in the world of CAR cellular medicine.
In the next post, we will continue this journey of cellular medicine by taking a deep dive into some more recent members of CAR world: CAR-Mo/Mac, CAR-gamma-delta T cells, and CAR-Tregs.
If you haven’t subscribed yet, please do so now. Subscribers receive every post of SciX newsletter directly in their inbox and an opportunity to ask questions and submit suggestions !
Why we need New CARs ?
Despite the success of CAR-T therapies, several challenges remain. Manufacturing of CAR-T cells is an expensive, lengthy and technically demanding process. It relies on patient’s own cells (autologous) as the source material, as many patients are often heavily pre-treated and lymphopenic (a lower than normal number of lymphocytes in the blood), many times they don’t have sufficient cells to yield a viable product or need more time in production. These technical limitations in turn further increases the cost of the product and on top of it patient looses crucial time to receive a life-saving treatment. Other challenges are the harmful side effects associated with CAR-T therapy like cytokine-release syndrome (CRS) and neurotoxicities. Besides, the clinical benefit of autologous CAR-T has been limited to a fraction of patients and a few indications, and, to date, have not shown similar activity in solid tumors thus highlighting the need for new strategies. Besides, to reduce the cost of cellular therapies, there is a huge demand of off-the-shelf allogeneic therapies. Though companies like, Allogene Therapeutics its partner, Servier, along with Adaptimmune and other companies are developing allogeneic CAR-T therapies, there is still a risk of GvHD and T-cell associated CRS and ICANS.
Leveraging cells of the innate immune system — Natural Killer (NK) cells, which possesses unique ability to recognise and kill transformed cells is an attractive option. Additionally, clinical trial results from six academic clinical studies conducted with allogeneic non-engineered NK cells for the treatment of 103 patients with R/R AML reported a 34% response rate. These data demonstrate the inherent anti-cancer activity of the body’s NK cells, and support the opportunity for increasing the activity of NK cells through the addition of a chimeric antigen receptor (CAR).
Why is CAR-NK an attractive option ?
NK cells are the body’s first line of defense against virally infected and tumor cells with an intrinsic ability to identify and eliminate transformed cells. They recognise their targets in a human leucocyte antigen (HLA)-unrestricted manner and can distinguish between healthy and transformed cells via recognition of self-major histocompatibility complex (MHC) class I molecules. Unlike T cells, they have an inherent ability to target tumor cells without genetic alteration. NK cells sense abnormal cells, such as tumor cells, which down-regulate MHC class I molecules in an attempt to escape T cell responses, and up-regulate ligands that are induced by DNA damage or malignant transformation. Upon forming immunological synapses with targets, NK cells elicit a potent response through the release of cytolytic granules and cytotoxic cytokines. This unique way of recognizing transformed cells makes NK cells an attractive candidate and since they do not cause GvHD, they are suited for allogeneic or off-the-shelf immunotherapy.
NK cells can also kill cells through antibody-dependent cellular cytotoxicity (ADCC), where they recognize antibody-coated target cells via CD16, an activating receptor that binds to the Fc portion of IgG antibodies. Once activated through CD16, NK cells are able to destroy these cells and secrete cytokines, such as interferon gamma, to recruit and potentiate adaptive immune cells, including T cells. This mechanism of killing is why NK cells are believed to be important for treating several human tumor types.
Besides, NK cells can be produced from a various sources (like Peripheral Blood Mononuclear Cells, cord blood, induced Pluripotent Stem Cells, Haematopoietic Stem and Progenitor Cells, immortalised cell lines) without relying on patient-specific immune cells. This will enable rapid, large-scale production and master cell banks.

Additionally, NK cells have a very safe profile, they do not cause CSR and neurotoxicity, this can save huge amounts of cost as patients can be dosed in an outpatient center. In contrast, with an allogenic CAR-T, to mitigate the risk of Graft-versus-Host Disease (GvHD) an additional TCR gene editing step is prerequisite. This additional gene editing increases the cost, complexity and the associated risk of gene editing errors.
Biotech CAR-NK Companies
NK cells show a rapid and potent anti-tumor cytotoxic activity however they do not typically expand upon encountering with antigens and lack the long-term persistence in the host. NK cells are short lived, with an average lifespan of 2 weeks in circulation. Moreover, NK cells are quite refractory to standard genetic manipulation techniques. Biotech companies are using multiple approaches to address these shortcomings and so far ongoing clinical trials of CAR-NK has shown positive and encouraging interim data.
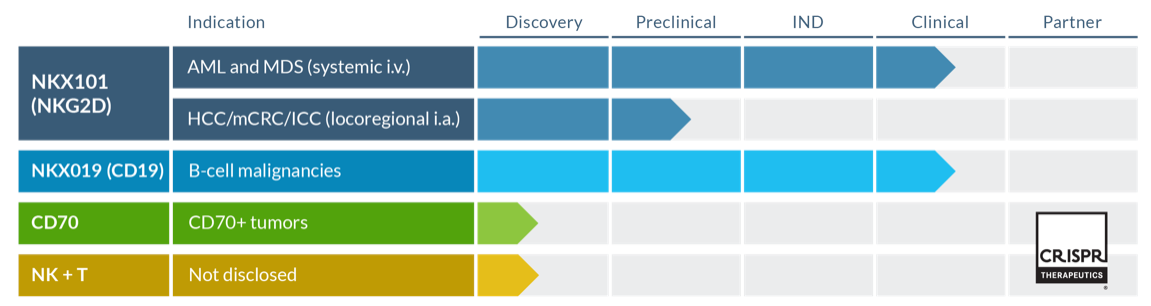
1. Nkarta (NASDAQ GS: NKTX, M. Cap= US$ 615M)
Nkarta is a clinical-stage biopharmaceutical company with two lead assets in clinical trials, NKX101 and NKX019. Other two assets are being developed in collaboration with CRISPR Therapeutics.
The company uses healthy donor PBMCs as a source of NK cells. Peripheral blood-derived NK cells are mature, highly functional and cytotoxic. Although NK cells represent only 5%-10% of peripheral blood, henceforth company performs an additional ex vivo expansion to increase NK cell numbers. This is done by co-culturing PBMCs with genetically modified irradiated K562 feeder cells. After 7 days, the frequency of NK cells reaches more than 90% . In about 10 days, Nkarta can generate greater that 10^8 NK cells/kg. However with donor derived PBMCs there will always be inter-donor variability.
After expansion, NK cells are retroviral transdused (>70% transduction efficiency) to express the CAR consisting of NKG2D ectodomain, co-stimulatory signaling motifs, and a membrane-bound form of IL-15 to improve the recognition of cancer cells and in vivo persistence of NK cells (NKX101) . mbIL-15 serves as an autocrine growth factor for NK cells, potentially obviating the need for systemic cytokine support.
NKG2D is the dominating activating receptor natively expressed on NK cells. It can bind to highly diversified ligands (MICA, MICB, ULBP1-6) which are poorly expressed on normal cells but can be induced on damaged, transformed or infected cells. Several cancer like leukemia, bladder, breast, colorectal, myeloma, ovarian & pancreatic cancers demonstrate up-regulation of NKG2D ligands.
However, in order to evade the immune response most of the tumors shed NKG2D ligands and moreover, higher levels of soluble MICA/B is associated with poor clinical prognosis in patients. Thus, only focusing to up-regulate expression of NKGD2 on NK cells might not be a good strategy to achieve higher clinical response and long-term remissions in tumors expressing MICA/B stress ligands. Strategies should be designed to overcome ligand shedding and re-engaging NKG2D:MICA/B mediated tumor recognition. This strategy is used by Fate therapeutics to design CAR for FT536 (More details under Fate subsection).
NKX101 is in Phase 1 clinical trial (NCT04623944) for treating of Relapsed/Refractory AML and higher-risk myelodysplastic syndromes. R/R AML and high-risk patients with MDS have poor prognosis and limited treatment options. NKX101 has orphan drug designation from FDA.
NKX101 clinical trial: Key observations
Haplo-matched related donor derived PBMCs were used to generate NKX101.
3/5 patients showed complete clinical response with 2/3 showing minimal residual disease negative when treated 3 doses of 1.5B CAR-NK cells. This initial data is really impressive.
Before infusion with CAR-NK all patients underwent lymphodepletion
(Cyclophosphamide+Fludarabine). The job of this chemotherapy isn’t to fight the cancer, but rather to prepare the body for the modified NK cells. Though there were minimal side effects from the CAR-NK cell therapy, patients can show side effects from the conditioning chemotherapy, like myelosuppression and infections. Henceforth, multiple cycles of conditioning chemotherapy will be a limiting step in planning treatment regimen with CAR-NK cells of highly treated cancer patient.
Contrary to CAR-T, allogenic CAR-NK were well tolerated, no CRS, GvHD or neurotoxicity reported so far.
Will be interesting to follow the clinical trial for data on the persistence of NK cells and time in remission after treatment.
NKX019 is in Phase 1 Clinical trial (NCT05020678) for the treatment of patients with CD19-positive r/r NHL, CLL and B-ALL. In market, we have FDA approved CD19-CAR-T therapy, however their use is limited and a lot of patients develop Grade 3+ CRS (13-49%) and Grade 3+ ICANS (18-31%). Over 25% of patients require ICU admission. CAR-NK cells can be safe treatment option either alone or in combination with CAR-T cells. Proof-of-concept studies have shown some promising effects, CAR19-NK and CAR19–T cells together increased the cytotoxicity and decreased CAR-T induced side effects in xenograft tumor NSG mice models.
Combinatorial therapy utilising both innate and adaptive immune cells, CAR-NK and CAR-T, respectively can open new treatment avenues for heavily treated cancer patients.
Clinical trial: Key observations
NKX-019 demonstrated safe profile and rapid response, 3CR and 2PR among 6 patients with NHL treated with 3 doses of 1.0 billion cells.
Durability of at least 5months in one patient who received 3 doses of 300M cells (lower dose).
Nkarta’s scientific advisory team is strong, led by a leader in cell therapy research, Dario Campana, MD, PhD and a recognised expert in NK cell biology, Lewis Lanier, PhD.
2. Fate Therapeutics (NASDAQ: FATE, M.Cap: US$ 2B)
Fate is a clinical-stage biopharmaceutical company, they have proprietary iPSCs platform to create genetically engineered, clonal master iPSC lines having preferred biological properties. Their iPSC platform is supported by an IP portfolio of over 90 issued patents and 100 pending patent applications.
iPSCs are an appealing source for NK cells given their clonal growth and high expansive ability as well as their capability to differentiate in vitro, allowing for homogenous NK cell products. However, iPSC-derived NK cells express low levels of CD16, which can be mitigated through genetic engineering. Another concern with iPSCs is that they may harbour ‘epigenetic memory’ i.e., DNA methylation signatures consistent with their somatic tissue origin. This can influence the development of specific cell lineages that differ from the donor cell.
Besides Fate therapeutics, iPSCs are also used by Allife Medical Science and Technology, China to generate CAR-NK cells.
Currently Fate has robust pipeline of multiplexed-engineered NK programs, with 5 of its CAR-NK assets: FT516 (NCT04023071), FT596 (NCT04245722), FT538, FT576 (NCT05182073) and FT536 assets in Phase 1 trail.
All of these assets are derived from a clonal master iPSC line and have been engineered to express a novel high-affinity 158V, non-cleavable CD16 (hnCD16) Fc receptor, which has been modified to prevent its down-regulation and to enhance its binding to tumor-targeting antibodies. This modification augments ADCC killing function of NK cells. Besides, Fate employs multiple functional modifications via CRISPR/Cpf1 in iPSCs derived NK cells to augment their activity, persistence, metabolic fitness and anti-tumor functionality.
CD38-KO iPSC cells are used to generate FT536, FT576, FT538. Elimination of CD38 increases the metabolic fitness of NK cells in high oxidative stress environments (e.g., suppressive tumor microenvironment) and prevents anti-CD38 monoclonal antibody-induced fratricide in case of combinatorial treatment with Daratumumab (anti-CD38 monoclonal antibody). Furthermore, CAR constructs in FT536, FT576, FT596, FT538 are engineered to express IL-15/IL-15 receptor fusion protein that enhances NK cell persistence.
Additionally to augment tumor recognition and anti-tumor activity of NK cells, FT536 contains a proprietary CAR that targets the α3 domain of MICA and MICB that boosts recognition of tumor cells, α3 domain targeting prevents MICA/B shedding, augments surface density & resists sMICA/B inhibition; FT576 contains anti-BCMA CAR; FT596 contains anti-CD19 CAR.

Summary of all FATE’s Phase 1 clinical Trials
More than 200 patients have been treated with off-the-shelf, multi-dose treatment paradigm in Phase 1 clinical trials showing substantial therapeutic benefit.
Interim P1 Clinical Data from FT516-101 Trial in r/r B-cell lymphoma (presented in 63rd ASH Annual Meeting, 2021):
No CRS, ICANS, GvHD in patients after up to two cycles of treatment, with each cycle consisting of 3 days of conditioning (Cy / Flu) and 1 dose of rituximab (375 mg/m2) followed by 3 once-weekly doses of FT516 with IL-2 cytokine support.
18 patients were treated with ≥90M cells/dose, 11/18 showed an objective response with 11 patients (61%) remained in the ongoing response at 3 months from initiation of treatment.
Ongoing P1 studies of FT516 and FT538 as monotherapy for AML patients:
12 patients with AML and a poor prognosis were enrolled. Objective response was observed in 3/9 patients treated with FT516 (3 at 90M cells/dose and 6 at 300M cells/dose) and 1/3 treated with FT538 (100M cells/dose).
Durable remissions >6 months were achieved in 2 FT516 patients without any additional therapeutic intervention.
Interim Phase 1 Clinical Data from FT596-101 Trial in r/r B-cell lymphoma (presented in 63rd ASH Annual Meeting, 2021):
Up to two cycles of treatment, with each cycle consisting of 3 days of conditioning (Cy / Flu) and 1 dose of rituximab (375mg/m2) followed by a single dose of FT596 (90M and 300M cells) without cytokine support.
No Dose-limiting Toxicities, and No Adverse Events of Any Grade of ICANS or GVHD were observed, infrequent, low-grade, and of limited duration CRS was observed in n=3 patients.
13 of 19 patients (68%) achieved OR (n=7/9 in monotherapy arm; n=6/10 in combination arm), including 3 of 5 patients (60%) previously treated with auto CD19 CAR T-cell therapy.
10 of 11 patients treated with a second FT596 cycle continue in ongoing response, with 3 patients in ongoing complete response at ≥6 months follow-up; Additional 2 patients reach 6 months in complete response.
Besides these ongoing trials, there are several clinical trials enrolling patients and we will closely watch the interim findings of these trials. If you are interested in learning more about the trial design, click this link.
Fate has collaborations with leading strategic, partners, key researchers, and top medical centers, which help them to accelerate the development of first-in-class product candidates and proprietary iPSC platforms and also provide them strong financial support.
3. Cytoimmune Therapeutics (Private; US$ 36.5M raised in 2021)
Founded in 2017, Cytoimmune is a clinical-stage biopharmaceutical company, developing proprietary CAR engineered NK cells (CAR-NK™) technology, which is licensed from the laboratories of Drs. Michael Caligiuri and Jianhua Yu at the City of Hope National Medical Center.
They use cord blood as a source of NK cells. Cord blood has many advantages, it is readily available through global cord blood banks and is highly proliferative, though there can be inter-donor variability. Cytoimmune conducts out a proprietary umbilical cord selection assay that which excludes 70-80% of the starting material and predicts both the quality and quantity of NK cells in final products. This screening step will ascertain the homogeneity of the final product and will minimise batch to batch variability.
Cytoimmune uses proprietary NK cells education and activation with feeder cells and perform multiple edits to generate tumor-reactive natural killer (TRACK-NK). TRACK-NK cells with the expression of key activation markers (NKG2D, NKp30, NKp44) and PDL1 are selected and expanded in proprietary manufacturing facilities. NK cells obtained from umbilical cord blood with ~50% transduction efficiency. Even without expression of CAR, these TRACK-NK have superior ability to efficiently migrate to tumor, secrete cytokines and kill tumor cells compared to non-engineered NK cells.
Human NK cells need endogenous IL-15 to develop, survive, expand and activate against tumor cells. To boost the activity and persistence of NK cells, TRACK-NK cells are engineered to incorporate a soluble, secretable form of IL-15 into the CAR construct (CYTO-NK-203, with CAR directed against PSCA), and the secretion of a bispecific killer engager (BiKE) molecule (CYTO-NK-301), enabling both dual antigen targeting and broad immune stimulation in one engineered cell.
In case of non engineered NK cells, membrane CD16 is shed after one ADCC-mediated killing. To enhance the killing efficacy of NK cells, cytoimmune proprietary CAR-NK cells (CYTO NK-102) are engineered to express high levels of CD16 receptor and enhanced ability to cycle CD16 on NK cell surface which is needed for ‘serial killing’.
Cytoimmune assets are backed by solid science but all are in early preclinical or manufacturing stages. The company plans to focus of increasing the transduction efficiency and yield per run to reach to pivotal readiness in 2024. A recent press release announced dosing on first patient with NSCLC in CYTO-NK-102 Ph1 clinical trial. The trial will enroll approximately 21 patients with relapsed or difficult-to-treat NSCLC (NCT05334329).
4. Glycostem (Private; raised funding in 2019)
Glycostem is a Netherlands-based clinical-stage biopharmaceutical company developing NK (oNKord) and CAR-NK (viveNK) allogeneic products in their proprietary and GMP-compliant platform technology, which is currently protected by nine patents. They use CD34+ hematopoietic stem and progenitor cells as source, they first expand these cells to derive fully functional, high-quality NK cells in large numbers (upto 50,000 fold). The technology platform is based on the use of closed bioreactor systems along with a proprietary synthetic, feeder cell-free, cell culture medium and a patented combination of growth factors. This enables off-the-shelf, safe, and low production cost products. They are using lentiviral transduction for CAR insertion into NK cells.
oNKord received an orphan drug designation for AML from both the EMA (2014) and FDA (2016). Ph1 clinical trial of oNKord in AML patients (N=10) demonstrated excellent safety profile and strong indication of clinical efficacy. Year 1 survival data showed 80% compared to population data showing 35% for the group with no intervention. Currently Glycostem is collaborating with various partners to develop CAR-NK cells, viveNKTM for solid tumors.
5. Immunity Bio & NantKWest (NASDAQ: IBRX, M. Cap= US$ 2.2B)
In 2020, ImmunityBio, a privately-held immunotherapy company, and NantKwest, Inc., a clinical-stage, NK cell-based therapeutics company announced a stock-for-stock merger. Now, ImmunityBio (IBRX) has a broad, clinical-stage pipeline – including 13 assets in clinical trials. Company has 2038+ worldwide patents extending to 2035 and beyond.
They used NK-92 cells as a source for engineering CAR-NK cells. NK-92 is the first NK-cell-based immunotherapy to receive IND approval by the US FDA for clinical testing. It is a homogeneous, immortalised NK lymphoma cell line that can be expanded ex vivo to achieve large cell numbers. They also lack expression of most KIRs, inhibitory receptors, which makes them even more attractive for cell therapy. However, they lack CD16 expression which is required for ADCC-mediated killing. To enhance ADCC potency of NK-92 cells, these cells were transduced with high-affinity CD16a-V158 mutant that is resistant to ADAM17-mediated cleavage and shedding, modified cells are known haNK. It is now in clinical trials against breast cancer (NCT03387085), Merkel cell carcinoma (NCT03853317) and squamous cell carcinoma (NCT03387111). IL15 is needed for NK cells to expand and for long-term persistence in circulation. Company’s proprietary lead antibody cytokine fusion protein, Anktiva is a IL-15 superagonist (IL-15 cytokine fusion protein). Multiple Phase I and Phase II trials in both liquid and solid tumors in over 700 patients have shown promising findings. In addition, Anktiva is in late-stage clinical trials for multiple solid tumors, including lung cancer, pancreatic cancer, TNBC, and glioblastoma, along with check-point inhibitors, chemotherapy, cell therapy and other immune stimulating agents.
Company is also employing CAR technology on haNK cells, the t-haNK platform, which can induce three modes of killing: innate, antibody-mediated and CAR-directed killing. Their PD-L1 t-haNK, recently cleared to start Phase II testing, and CD19 t-haNK, cleared to start phase I testing, other assets in pipeline, including HER2, which is nearing IND submission, and EGFR, which is advancing through clinical enabling studies, among others, will enable to potentially address an even broader range of cancers as part of a chemotherapy-free combination regimen. They have also initiated a phase I clinical study against PD-L1 expressing non-small-cell Lung Cancer. The P2 QUILT 88 trial (NCT04390399) of anti PDL1-t-haNK cell therapy combined with IL15 superagonist, N-803 and DAMP inducers more than doubled historical overall survival with 3rd to 6th line advanced Pancreatic Cancer.
The company is also developing Memory Cytokine Enriched Natural Killer Cells—M-ceNK (Allogenic / Autologous). First-in-human subjects dosed with M-ceNK in 2022 (NCT04898543).
6. Catamaran Bio (Private; raised US $42M in 2020)
Catamaran is a biotechnology company developing off‑the-shelf NK cell therapies to treat cancer. It has developed TAILWIND™, a proprietary and integrated suite of technologies, to engineer, expand and process NK cells into safe and effective, off-the-shelf cell therapy products for multiple cancer types. They are pioneering the use of non-viral NK cell engineering with the TcBuster Transposon System to efficiently produce potent CAR-NK cell therapies. Transposon system can allow the delivery of large genetic payloads and multiplex cell engineering can be carried out in single step. They generate NK cells with tumor microenvironment switches, like TGFβ, an immunosuppressive cytokine found in a majority of solid tumors. Company is also focussing on optimising CAR architecture for optimized function in NK cells for improved cell activation and potency-boosting switches to address the hostile TME.
They are focusing on development of two allogeneic CAR-NK assets: CAT-179: HER2 CAR-NK Cell Therapy for breast cancer and gastric cancer (CAR targeting HER2, combined with an IL-15 cytokine, and a TME-switch that neutralizes the effects of the TGFβ immunosuppressive signal) and CAT-248: CD70 CAR-NK Cell Therapy for renal cancer (CAR that targets CD70; an IL-15 cytokine, a TME-switch for TGFβ immunosuppressive signal, and the elimination of CD70 expression to enable scalable manufacturing).
Catamaran is financially supported by a syndicate of leading life science investors.
7. Wugen (Private; raised US $172M in 2021)
Wugen is a clinical-stage biotechnology company developing a pipeline of allogeneic cell therapies to treat a broad range of hematological and solid tumor malignancies. They are developing memory NK cells with their proprietary Moneta™ platform. Cells are expanded without the use of feeder cells and are cryopreserved to generate an off-the-shelf product.
Memory NK cells are hyper-functional, long-lasting immune cells that exhibit enhanced anti-tumor activity and a cytokine-induced memory-like (CIML) phenotype in immunosuppressive solid tumor microenvironment. Currently, company has one mNK asset, WU-NK-101, which has shown promising efficacy in preclinical studies. When used in combination with monoclonal antibodies in preclinical models, WU-NK-101 demonstrated further enhanced anti-tumor activity, with robust tumor penetration and persistence.
8. Artiva Biotherapeutics (Private; raised US $120M in 2021)
Artiva generates NK cells from healthy donor umbilical cord blood (UCB) units, which are selected for key characteristics. With their AlloNK Platform they can generate thousands of doses of pure, cryopreserved, infusion-ready NK cells from a single UCB sample.
Our internal programs are currently focused on specific targeting of CD20 (AB-201) and CD19 (AB-202) in B-cell lymphomas and HER2 in solid tumors. On Sept, 2022 U.S. FDA has cleared the company’s IND application for AB-201.
They have another asset, AB-101 which is a highly active allogeneic NK cell therapy being developed for therapeutic use in combination with a variety of targeting modalities, including monoclonal antibody therapy and NK-engager bispecific technology. They have an ongoing Phase 1/2 clinical trial (NCT04673617) of AB-101 alone and in combination with rituximab (the anti-CD20 monoclonal antibody) for r/r B-cell NHL who have progressed beyond two or more prior lines of therapy.
Atriva is supported by leading life science investors and corporate partners. The GC family of companies has invested significantly in state-of-the-art research and GMP manufacturing for the development of cell therapies.
9. Athenex (Kuur Therapeutics)(NASDAQ: ATNX, M. Cap=US$30M)
Kuur Therapeutics was acquired by Athenex, Inc., (NASDAQ: ATNX), on May 4, 2021. The company focusses on developing a wide range of CAR-NKT. Natural Killer T cells (NKT) are rare immune cell subtype that shares some characteristics of T and NK cells, partly in the belief that their unchanging TCR supports an allogeneic approach.
Company’s CAR-NKT cell product candidates include both autologous (KUR-501) and allogeneic (KUR-502 and KUR-503) products. KUR-501 is an autologous cell therapy targeting GD2, a molecule expressed on almost all neuroblastomas, and on some other tumors, with restricted expression on normal tissues. KUR-501 is also designed to address key limitations of current CAR immune cell therapies by secreting the cytokine IL-15. The Ph1 GINAKIT2 clinical trial (NCT03294954) is open and recruiting patients. Interim findings from KUR-501 trial of ten evaluable patients with escalation to a dose level (DL) of 1×10^8 cells/m2, resulted in 1-CR and 1-PR and 3-SD. Tumor biopsies show CAR-NKT cells homing to the neuroblastoma tumor site at all dose levels. KUR-501 has so far demonstrated a promising safety profile, with only one case of grade two CRS and no cases of immune effector cell-associated neurotoxicity syndrome (ICANS).
KUR-502 is an allogeneic CAR-NKT cell therapy targeting CD19 for the treatment of relapsed, refractory DLBCL, adult ALL and CLL. The Ph1 ANCHOR clinical trial, is currently open to recruitment. In the ANCHOR study, two patients were evaluated when the interim findings were released, lymphoma patients were treated at the starting DL of 1×10^7 cells/m2, one patient experienced a CR and the other a PR. Biopsy of the patient’s lymph node prior to conversion to CR status revealed viable, allogeneic CD19 CAR-NKT cells. The patient with a PR had previously failed autologous CAR-T cell therapy. KUR-502 has so far demonstrated a promising safety profile with no CRS, no ICANS, and no evidence of GvHD. Other allogeneic asset, KUR-503 is CAR-NKT targeting GPC3 for the treatment of patients with advanced hepatocellular (liver) carcinoma. Company is planning to file the IND in 2H2022.
Clinical trials are conducted in conjunction with company’s partners at Baylor College of Medicine in Houston, Texas, USA.
10. Sanofi SA (NASDAQ: SNY, M.Cap = 108.5B)
Sanofi, multinational pharmaceutical and healthcare company, the pharma giant, acquired Kiadis Pharma in 2021 for US$358M and have patterned with Scribe Therapeutics to extent their oncology pipeline to CAR-NK cell therapy. Sanofi has non-exclusive rights to Scribe’s proprietary CRISPR platform (which includes suite of custom engineering genome editing and delivery tools called CasX-Editors) of wholly owned enzymes for creating ex vivo NK cell therapies. CasX enzymes protein is less than 1,000aa, compared with the 1,200–1,400aa size of Cas9 which makes them more capable of delivering CRISPR packaged in a viral vector compared to Cas9.
Currently, Sanofi’s CAR-NK pipeline includes SAR445419 (formerly KDS1001), an NK cell therapy candidate acquired from Kiadis Pharma. SAR445419 is being developed for r/r AML that is under study in a Phase I trial.
11. M.D. Anderson Cancer Center (MDACC)
Academic Institute, funded by MoonShot Program, Sally Cooper Murray Endowed Chair in Cancer Research, and the National Institutes of Health
Researchers at The University of Texas MD Anderson Cancer Center are developing new approaches to engineer NK cells into efficient, persistent CAR-NK cells. They conducted initial clinical trials with CAR-NK targeting CD19 with immunocytokine IL-15, for non-Hodgkin’s lymphoma and chronic lymphocytic leukemia patients. Data released in 2020 from 11 patients showed eight responding to the therapy, including seven in CR. After a median of 13.8 months’ follow-up, patients continued to show no evidence of disease, and five received post-remission therapy. Findings were outstanding and soon after Takeda in licences the CAR-NK asset, which is now in Phase 2 clinical trial (see Takeda’s section for more details).
Another Phase 1/2 clinical trial (NCT03056339) conducted by MDACC is based on UCB-NK cells transduced with a CD19-targeting scFv, interleukin 15 (to enhance NK cells persistence) and an inducible caspase-9 (iC9) suicide gene as a failsafe mechanism. Preliminary results demonstrated that the approach is safe, despite only partial HLA matching between donor and recipient, and potency is high (seven out of 11 patients achieved complete remission). Of note, CAR.19/IL-15/iC9-NK cells were detected at low levels up to 12 months after the beginning of the treatment, whereas they normally disappear within 2 weeks. Their another clinical trial with CAR.19/IL-15/iC9-UCB NK Cells, in high-dose chemotherapy, and stem cell transplant in treating participants with B-cell Lymphoma has been withdrawn due to lack of funding (as stated on clinicaltria.gov)
MDACC researchers have recently published promising preclinical findings of next generation CAR-NK, KIR-based inhibitory CARs. These Next generation CARs are designed as a dual-CAR system incorporating both an activating CAR against the cognate tumor antigen and an NK self-recognizing inhibitory CAR that transferred a ‘don’t kill me’ signal to NK cells upon engagement with their TROG+ siblings. So, trogocytosis is a newly discovered mechanism by which surface material is transferred from targeted (tumor cell) to effector cells (NK cells). This active process dampens the cytotoxic response of NK cells and induce fratricide and finally tumor escape immune response. This research provides a further understanding of CAR NK biology and suggests that clinical evaluation of the dual-CAR strategy is warranted.
12. Takeda Pharmaceutical (NASDAQ: TAK, M.Cap=41B)
Takeda, Tokyo-based pharmaceutical giant has currently two CAR-NK assets in pipeline with targets disclosed - TAK-007 (in-licensed from MDACC), CAR-NK targeting CD19 for treatment of non-Hodgkin’s lymphoma and chronic lymphocytic leukemia, and the second one targeting the antigen BCMA in multiple myeloma. Takeda is also interested to develop the two other lead CAR-NK programs with MDACC for solid tumors, breast cancer brain cancer glioblastoma. Phase 2 clinical trial (NCT05020015) of TAK-007 in patients with r/r B-cell Non-Hodgkin Lymphoma is ongoing.
13. Affimed (NASDAQ: AFMD, M.Cap = US$261M)
Affimed is a clinical-stage biotechnology company focussed to generate innate cell engagers (ICE) on their proprietary ROCK (Redirected Optimized Cell Killing) platform. They have broad pipeline of wholly owned and partnered ICE molecules (AFM13, AFM24, AFM28 and AFM32) as monotherapies, as well as in combination with NK cells and other I-O therapies. These ICE molecules bind CD16A to a differentiated epitope, this gives it added benefit to activate NK cells and also macrophages without a co-stimulatory signal. Also there is no dilution and sink effect of these engagers through neutrophils which predominantly are CD16B+. Henceforth, preclinical studies have also shown that Affimed’s ICE molecules demonstrate higher cytotoxicity compared to conventional and Fc-enhanced antibodies and also are efficient against tumors with low antigen expression.
AFM13 is a first-in-class ICE molecule designed to target CD30+ lymphomas. AFM24 is an EGFR-binding ICE molecule that kills EGFR-expressing tumors. AFM28 targets CD123 positive tumors, and AFM32 is in partnership with Roivant, other novel ICE is Genentech-partnered molecule which are all at the pre-IND stage.
Phase 1/2 clinical trial with modified UCB derived NK cells (pre-activated with IL12/15/18, expanded with Universal Antigen Presenting Cells, K562 feeder cells) in combination with the antibody AFM13 (AFM13-NK) and AFM13 alone in patients with CD30 positive R/R Hodgkin lymphoma or non-Hodgkin lymphoma is currently recruiting patients. This trial is in collaboration with MDACC. Positive interim data on 19 patients presented at AACR 2022. Across all dose levels 17/19 patients responded (89% ORR) with 10 CRs and 7 PRs. Patients were heavily pretreated with median 7 lines of prior therapy. 7/8 patients with CR remain in CR at median follow-up of 6.5 months.
14. PearsonGen BioTherapeutics (Private; raised US $31.4M in 2022)
PersonGen (Suzhou, China), has registered 13 CAR-T and CAR-NK clinical trials on Clinicaltrials.gov, and officially launched clinical trials by collaborating with more than 10 medical centers in China.
Their anti-CD19 CAR-NK cells are developed from NK-92 cell line engineered to contain anti-CD19 attached to TCRzeta, CD28 and 4-1BB signaling domains is in clinical trial (NCT02892695).
Besides PearsonGen BioTherapeutics, around 13 clinical trials of CAR-NK cells are ongoing in China sponsored by following: Xinqiao Hospital of Chongqing; Asclepius Technology Company Group; Allife Medical Science and Technology; Zhejiang University; Wuhan Union Hospital; Second Affliated Hospital; School of Medicine, Zhejiang University.
Other players of CAR-NK biotech market are GC Bio Pharma and VaxCell Biotherapeutics of South Korea, Century Therapeutics (NASDAQ: IPSC), Celularity (NASDAQ: CELU), Dragonfly Therapeutics, Acepodia, iCell Gene Therapeutics, Senti Biosciences (NASDAQ: SNTI), Shoreline Biosciences, etc.
The global NK cell therapeutics market is expected to reach US$ 5,676.1 million in 2032 from $297.2 million in 2024 at a CAGR of 44.59% during the forecast period 2024-2032.
Lessons Learned from CAR-NK Trials
CAR-NK cell therapy have safe treatment profile, contrary to CAR-T cells they do not mediate severe toxicities like GvHD or CRS and, therefore, do not require stringent HLA matching.
They are safe in allogeneic setting and can be dosed outside of hospitals which will save cost and time.
There is not enough data from clinical trials of CAR-NK cells against solid tumors, but so far allogeneic CAR-NK cells are safe, no GvHD, CRS or ICANS and more importantly patients with advanced PDAC and glioblastoma have shown encouraging overall outcomes.
NK cells are quite refractory to standard genetic manipulation techniques, which resulted in major delays of the first clinical trials with CAR-NK cell. However, both the gene editing engineering and general delivery system are advancing at a rapid pace and in no time this technical issue will be resolved.
The limited proliferation potential of NK cells usually does not allow them to persist longer than 2–3 weeks after injection in vivo. Because of this, multiple doses (2-3) with lot of NK cells (300M to 1.5B) and even multiple cycles for some patients are needed for effective treatment. Before each cycle of CAR-NK therapy, patient has to undergo conditioning chemotherapy which comes with its own deleterious effects. It should be considered, because many patients recruited under these trials have already dampened immune sytstem and heavily treated.
Early efforts in providing NK cells with stimulatory cytokines prolonging half-life like IL-15 and increasing expression of chemokine receptors, or depleting inhibitory receptors and dual CAR models are encouraging and worth being further tested in clinical trials either alone or in combination with other therapies.
Since NK cells killing mechanism is based on ADCC and is HLA unrestricted, CAR-NK holds promise to treat all patient subtypes with a more acceptable safety profile compared with current advanced treatments.
Concluding Remarks
NK cell-based therapy is well positioned as a safe off-the shelf anti-tumor therapy, however many technical challenges remain open. There is huge need for development of optimal methods for expansion and cryopreservation of NK cells to ensure that high quality of the product is sustained and the therapy is cost-effective. Furthermore, advancement in CAR designing to enhance NK cell potency, long-term NK cell persistence and expansion are needed for successful long term remission in patients with solid tumors.
In conclusion, NK cells are first line of defence, they have killer instincts, CAR-NK candidates in early clinical studies proves to be a safer cell therapy that can be dosed outside of hospitals, if it proves itself in advanced clinical trials, it will have a shot in the mainstream world of cellular therapy.