Dear Readers,
This article will explore another interesting CAR-T innovator company—ADAP ! If you are new to SciX newsletter and want to get a view of what to expect in the articles, please click here.
16. Adaptimmune Therapeutics (NASDAQ: ADAP, M.Cap US$ 860m)
Adaptimmune is a clinical stage biopharmaceutical company focussed on developing an industry-leading T-cell therapy for multiple solid tumor indications. James Noble founded the company and served as its CEO for 20 years before stepping down on Sept 1, 2019 to be replaced by chief financial officer and former GlaxoSmithKline executive Adrian Rawcliffe. The company had seen a turbulent couple of years for its T-cell receptor (TCR) therapies, punctuated by mixed readouts in clinical trials but GSK stepped in with $350 million alliance which is still actively involved in pushing through a leading program in the TCR field. The company is now big, advancing TCR research for multiple solid cancers with its operations in the UK and the USA.
The company had some great scientific co-founders like Dr Bent Jakobsen. Dr. Jakobsen has authored numerous scientific papers and is considered a world expert in the field of T cell receptor immunology. Another scientific co-founder Dr Jo Brewer, serving as Senior Vice President in the company is leading the Allogenic Research Platform & brings in 18 years of Immunology expertise. Company’s foundation of TCR therapy started with NY-ESO SPEAR T cells, which was transitioned to GSK in 2018. The company made waves when it showed activity of SPEAR T cells in synovial sarcoma and then there was a big move in stock in May last year when the company announced responses achieved in lung, head and neck, esophagogastric junction or EGJ, liver cancer. However, in 2019 there were concerns over their now most advanced asset (afami-cel) regarding its toxicity when its Phase 1 study saw a treatment-related death due to pancytopenia/aplastic anaemia. As the efficacy data from more patients came in, it was clear that the efficacy was durable with strong anti-tumor response and just like all other cell therapies, afami-cel carries significant but controllable toxicities (discussed later in detail). Based on the top line data, company is planning to file BLA in 2022 and hopefully afami-cel could become the world’s first approved engineered T-cell receptor therapy.
AdaptImmune seems to be a front runner in the space, although Immunocore's TCR bispecific Tebentafusp could get approved for uveal melanoma given convincing overall survival benefit.
Advancements
SPEAR (Specific Peptide Enhanced Affinity Receptor) T‑cell platform
SPEAR T cells have modified hypervariable complementarity determining regions (CDRs) in order to enhance affinity to the cancer cell's HLA peptide complex.
Background on CDRs: Naturally occurring T-cell receptors consist of two associated protein chains: the alpha (α) and beta (β) chains. Each of the chains has two regions: a variable region and a constant region. The constant region sits next to the T-cell membrane and the variable region of the two chains binds to the target peptides. The variable region of each TCR chain has three hypervariable complementarity determining regions or CDRs.
Naturally occurring TCRs struggle to recognize cancer proteins because the cancer proteins appear to be very similar to other proteins within the body. To overcome this, TCRs are optimised to recognise a specific peptide derived from antigen which is specifically expressed on cancer cells and not on normal cells. Upon adoptive transfer of autologous SPEAR T cells (with engineered TCRs) in patients circulation, they continue to use physiological signaling pathways unlike CAR-Ts, so the risks for CRS and neurologic toxicity are expected to be lower than those reported in studies using CARs.
SPEAR platform allows to identify and select the TCRs which are likely to prove the most effective in patients whilst minimizing the risk of cross-reactivity to healthy and non-target tissue.
They generate TCR therapeutic candidates through a process which includes:
Deriving engineered TCRs capable of binding to any selected target cancer peptide
Using proprietary disulfide bond methodology to develop stable and soluble engineered TCRs
Selecting maximum potency and specific engineered TCRs from a large, diversified library by utilising proprietary phage display system.
Testing the selected TCRs for any cross-reactivity in pre-clinical studies
Watch this video to learn more about how SPEAR T cells work:
Allogenic SPEAR T-cell program
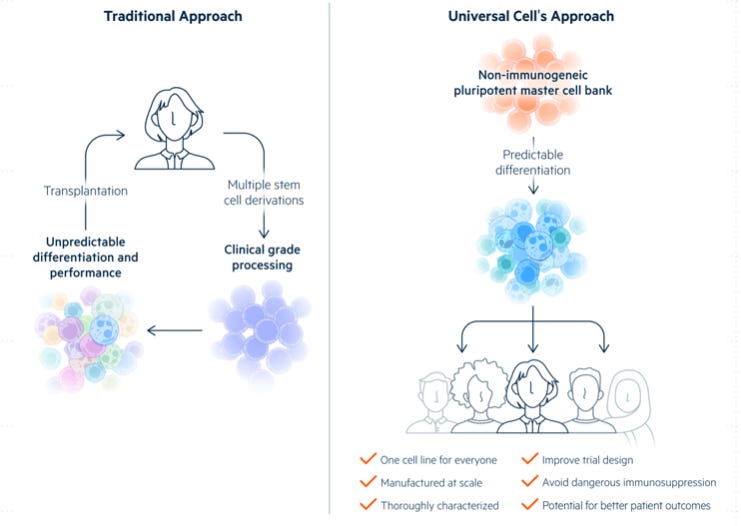
Generating T-cells using Human Induced Pluripotent Stem Cells (HiPSC) in vitro and then engineering them to express SPEAR T-cell Receptors (TCRs). In 2015 the company started working to make T-cells from edited stem cells in collaboration with a small gene editing company, Universal Cells (now an Astellas Company) in Seattle. The company was founded by one of the pioneer of genetic manipulation of human stem cells, Dr. David Russell in 2013.
Company uses Astellas’ Universal Donor Cell (UDCs) and Gene Editing Platform for generation of non-immunogenic off-the-shelf T cells. UDCs have major advantages as the precursor cells for the generation of allogenic T cells, like 1. No risk of rejection by host (GvHD), 2. Block lysis by Natural Killer cells, and 3. Enhanced safety, which enables in vivo elimination of implanted T cells. All these features in UDCs are achieved by genetic manipulation (either, elimination or addition) of specific genes in pluripotent stem cells:
The polymorphic HLA proteins (HLA-A, B and C) are edited out/eliminated
Specific non-polymorphic HLA molecules are edited, by adding single chain B2M:HLA-E fusion protein in the B2M gene locus.
Addition of suicide gene like Thymidine Kinase
These class I-engineered stem cells can serve as UDCs in applications where the differentiated cell product does not express HLA class II (macrophages, dendritic cells).
Adaptimmune’s allogenic team led by Dr. Jo has made tremendous progress in refining the protocol for generation of T cells from UDCs. Specialised stromal cells are needed by T cells to grow, still the field relies on “foreign” stroma cell lines, but Adaptimmune can make stem cells grow their own stroma, which may eliminate a potential source of contamination. Additionally, their T cells do not require human serum to grow–which is often used to provide growth factors and other elements. This further reduces the variability and contamination as these cells progress to the clinic.
In the video below, Dr. Jo provides an overview of the allogeneic SPEAR T-cell or “off-the-shelf” platform data presented at the annual American Cell & Gene Therapy conference in Washington DC.
HLA-independent TCRs (HiTs)
The company is also working on HLA-independent TCRs (HiTs) that can recognise targets without the need for the usual antigen-presenting proteins called HLA. The company will co-develop HiTs with Astellas.
Recently company announced preclinical data from its HiT targeting mesothelin, during a poster presentation at the ASGCT meeting. Data showed that the HiT program is CD8 and HLA-independent, also that it's not neutralised by soluble mesothelin (a problem for TRuCs and CAR-T).
Integrated Cell Therapy Capabilities
Company has invested in manufacturing innovation which drives 40% reduction in SPEAR T-cell therapy COGs; development of cell and vector manufacturing in house which reduces the costs by more than 90%.
Next Gen Products
Product Pipeline
The company has 5-year core value drivers: The “2-2-5-2” plan
Two marketed SPEAR T-cell products targeting MAGE-A4
Two additional BLAs for SPEAR T-cell products
Five autologous products in the clinic
Two allogeneic products entering the clinic
Autologous Platform
Allogeneic Platform
ADP-A2M4 (afamitresgene autoleucel or afami-cel, previously known as ADP-A2M4; MAGE-A4c1032)
First generation TCR directed towards MAGE-A4 antigen, which is the most commonly expressed cancer-testis antigen on several solid tumors.
Spearhead 1 trial started in December, 2019 to investigate the efficacy and safety of ADP-A2M4 (now afami-cel) in HLA-A*02 eligible and MAGE-A4 positive subjects with metastatic or inoperable (advanced) Synovial Sarcoma (Cohort 1 and Cohort 2) or MRCLS (Cohort 1) (NCT04044768). Data from Cohort 1 comprising of 45 patients will be used to support the BLA filing and Cohort 2 data also from 45 patients will strengthen the efficacy and safety database and will aid in descriptive sub-group analyses. Enrolment in Cohort 1 is complete, and Cohort 2 is currently recruiting.
It should be noted that afami-cel is designed to work only in patients with the HLA-A2 type, present in ∼50% of Caucasians, ∼40% of Hispanics, ∼20% of African Americans, and up to 20% of Asians. HLA haplotype matching is crucial with engineered TCR therapies, a concept somewhat analogous to matching blood groups in blood transfusions. To overcome this, company is also working on next gen TCRs-HiT platform pipeline.
Clinical Trial Data Timeline:
Company presented the updated data of Phase 1 data in CTOS, 2020 which demonstrated strong durable response with afami-cel in synovial sarcoma patients. Seven out of 16 patients (44%) had confirmed PR per RECIST criteria, with disease control in 15 patients (94%). There was a median duration of response of 28 weeks (range: 12-72+ weeks). The response rate was considerably superior to response rates observed with available second-line therapies in synovial sarcoma.
Then in ASCO Congress company presented the updated data of Phase 2 SPEARHEAD-1 trial which showed that afami-cel is efficacious and well-tolerated in heavily pre-treated patients, the overall response rate was 39.3% (13/33), 41.4% (12/29) for synovial sarcoma; 25.0% (1/4) for MRCLS. Two CRs in patients with synovial sarcoma and Disease control rate of 84.8% (28/33).
Improvements in sum of diameters over time have been observed. Median duration of response was not reached–range (weeks): 4.3+, 38.0+.
Afami-cel is the most advanced program & has entered a phase 2/3 clinical trial for patients with advanced synovial sarcoma and mixoid round cell liposarcoma (MRCLS), two types of rare cancers found more commonly in younger adults and children. The Company plans to file for a biologics license application (BLA) in 2022 for afami-cel, if approved, it would be the first TCR T-cell therapy.
In their translational work, they observed up regulation of PD1 (programmed cell death) after administration of T cells which led to SPEARHEAD-2 trial.
Spearhead 2 Phase 2 trial started in July 2020. This study will investigate the efficacy and safety of ADP-A2M4 in combination with pembrolizumab in HLA-A*02 eligible and MAGE-A4 positive subjects with recurrent or metastatic Head and Neck cancer (NCT04752358).
ADP-A2M4CD8
Second generation TCR directed to MAGE-A4, these cells also express the CD8α co-receptor alongside the engineered TCR that targets MAGE-A4.
The CD8α enhancement boosts the engagement of all CD4+ T-cells allowing them to perform their support functions* to their maximum potential, i.e. increasing TCR binding avidity and enhancing the polyfunctional response of CD4+ T-cells against tumor antigens.
*Adaptimmune uses CD4 T (T helper cells) and CD8 T (Cytotoxic T cells) in therapy development. Let’s briefly understand the function of each cell type:
CD8+ T cells do the primary function of killing infected or tumor cells through multiple mechanisms including expression of granzymes and perforin, as well as the secretion of cytokines such as interferon γ and tumor necrosis factor α.
CD4+ T cells have more subtle but very important support role, coordinating with other immune cells like dendritic cells and CD8+ T cells to initiate a more powerful anti-tumor response. A TCR needs help from what is called a ‘co-receptor’ to first ‘notice’ a tumor cell, and then fully interact with it.
The engineered CD4+ T-cells can effectively kill tumor cells, as well as stimulate DCs to mature (eg, through CD40L/CD40 interaction), upregulate co-stimulatory molecules on the cell surface (eg, CD80), and induce IL-12 secretion. These mechanisms in turn boost CD8+ T-cell activation, clonal expansion, and differentiation into effector and memory T-cells. ADP-A2M4CD8 may enhance tumor cytotoxicity via improved CD4+ T-cell effector and helper functions.

Company discovers TCRs that are naturally more suited to using the CD8 co-receptor, which means that CD4+ T cells were not be activated to their full potential in company’s first generation product ADP-A2M4. Although, there was activation of CD8+ T cell functions that benefit patients, most importantly killing the tumor, but maybe not the more subtle CD4+ help to improve communication and long-term effects of the product. Check out the AACR 2019 poster if interested to learn more about the activity difference of ADP-A2M4 and ADP-A2M4CD8.
Ongoing trial investigates the safety and tolerability of MAGE-A4ᶜ¹º³²T cell therapy in subjects directed towards a MAGE-A4 peptide expressed on tumors in the context of HLA-A*02 for urinary bladder, melanoma, head and neck, ovarian, non-small cell lung, esophageal, gastric, synovial sarcoma, or myxoid/round call liposarcoma tumors (NCT03132922).
Ongoing SURPASS trial initiated in Aug, 2019: This study will investigate the safety and tolerability of autologous ADP-A2M4CD8 T-cell therapy in subjects who are HLA-A2+ and tumor antigen status and whose urothelial, head and neck, gastric, esophago-gastric junction, non-small cell lung, or esophageal cancer that express the MAGE-A4 protein (NCT04044859).
On Sept, 2021, the company announced updated data from its Phase 1 SURPASS trial in multiple solid tumors, which was presented as a digital poster in European Society for Medical Oncology (ESMO) annual meeting. Early data is promising, majority of patients experienced anti-tumor activity with a disease control rate of 86%, see the table below for details. Next-gen ADP-A2M4CD8 T cells demonstrated efficacy and well-tolerability in heavily pre-treated patients across a broad range of tumor indications.
Additional data also showed post-infusion increase in a subset of the 22 measured serum cytokines, significant increase in serum IL-12 supporting dendritic cell engagement (i.e., a broader immune response).
Ongoing SURPASS-2 trial initiated in Sept 2021 in collaboration with ICON plc. This study will investigate the efficacy of autologous ADP-A2M4CD8 T-cell therapy in HLA-A2+ Subjects With MAGE-A4 Positive Esophageal or Esophagogastric Junction Cancers (NCT04752358).
ADP-A2AFP
This SPEAR TCR targets alpha-fetoprotein (AFP). AFP is an established clinical biomarker of Hepatocellular Carcinoma, it is highly expressed on the tumor and not in normal tissues.
A Phase I Open Label Clinical Trial is ongoing to evaluate the safety and anti-tumor activity of autologous SPEAR T cells for Alpha Fetoprotein (AFPᶜ³³²T) in HLA-A2+ subjects with advanced HCC (NCT03132792).
Early data from the trial was shared in International Liver Congress, 2020. The data demonstrated acceptable safety profile with no evidence of significant T-cell related hepatotoxicity and no protocol-defined dose limiting toxicities. Overall, nine patients were treated as of the data cutoff of July 6, 2020, of those four patients have been treated with ~5 billion or more transduced cells: 1 patient with the complete response, 1 with stable disease (SD), and 2 had progressive disease (PD). Watch the below video for more details:
ADP-A2M10
Affinity-enhanced autologous MAGE-A10c796T-cells directed toward MAGE-A10 in the context of HLA*02 (SPEAR T-cells). MAGE-A10 is expressed in ∼10–50% of urothelial, melanoma, head & neck, and non-small cell lung (NSCLC) cancers.
Ongoing 2 clinical trials (NCT02592577; NCT02989064), early data presented in ESMO, 2018.
Current Clinical Trials
Partners
Alpine Immune Sciences: collaborated to develop next-generation SPEAR T-cell products that incorporate Alpine’s secreted and transmembrane immunomodulatory protein (termed SIP™ and TIP™) technology.
Astellas: collaborated to utilise the novel HLA-independent TCR (“HiT”) platform and wholly-owned subsidiary Universal Cells, Inc, to bring new stem-cell derived allogeneic T-cell therapies to people with cancer.
R&D efforts at Adaptimmune in this area expanded following a January partnership with Universal Cells, a subsidiary of the Japanese pharma giant Astellas Pharma.
Cell and Gene Therapy Catapult: for vector supply for all three SPEAR T-cell therapies, ADP-A2M4 (MAGE-A4) ADP-A2M4CD8, and ADP-A2AFP (AFP).
GSK: In 2014, strategic collaboration for up to five programs including the first: NY-ESO SPEAR T-cell therapy program (transitioned to GSK in 2018) and second PRAME. The other three assets need to decided.
Noile-Immune Biotech: to develop SPEAR T-Cell Products expressing IL-7 and CCL19, incorporating PRIME (PRoliferation Inducing and Migration Enhancing) technology as a Next-Generation Treatment for Cancer Patients
Genentech, a member of the Roche Group: to develop and commercialize allogeneic cell therapies to treat multiple oncology indications. Adaptimmune will receive $150 million upfront, $150 million over the next five years in additional payments, and development, regulatory and commercial milestones payments potentially exceeding $3 billion in aggregate value, as well as royalties, across multiple programs.
CCIT, Denmark: to develop next-generation ‘supercharged’ TILs co-expressing IL-7
Key management
Ad Rawcliffe: CEO since Sept, 2019, He has over 20 yrs of experience within biopharmaceutical industry and previously served in GSK at varied senior roles.
The company has divided teams into: 1. Early stage development group; 2. Pipeline research team; 3. Allogenic Research and 4. Late Stage Development which are by experienced team leaders
Dr. Mark Dudley: Senior VP, leading the Early Stage Development group since 2019. He is the pioneer in the field of cell and gene therapies, while working in Novartis in 2013-2016 he contributed to BLA activities, enabling approval of first CAR-T, Kymirah. He has immensely contributed in scientific community with more than 100 publications which includes seminal work of demonstrating that lymphodepletion prior to adoptive cell therapy can mediate complete response in advanced refractory patients (Seminal Oncol, 2007). Credit also goes to him in advancing first-in-human clinical studies with young T cells, optimising TCR-T cells and next-gen T cells.
Dr Karen Miller: Senior VP, leading Pipeline Research team since 2019. She is an immunologist by training with more than 25 years of experience in drug discovery. She was in GSK, UCB Phama at senior role before joining Adaptimmune. Dr Miller did her PhD in cell secretory mechanisms from Liverpool University.
Dr. Jo Brewer: Senior VP, leading the Allogeneic Research team since 2018. Dr Brewer has more than 18 years experience in immunology research and she was one of the founding scientists at the company in 2008. Dr. Brewer holds PhD in Cellular Signalling from the university of Cambridge.
Dr. Dennis Williams: Senior VP of Late Stage Development team since 2019, he has 20 yrs of experience in the pharmaceutical and biotech industries. Dr Williams holds Doctorate in Pharmacy from University of Florida.
Financials
As of June 30, 2021, Adaptimmune had a Net cash position of $263m. The company is funded through early 2023. In their most recent quarter, they had a cash burn of around $40m. More than 50% of the shares are held by prominent institutions including a 6% stake held by Baker Brothers. 4% of the shares are short.
The stock is fairly liquid with an ADTV of around 2m shares. The stock seems fairly attractive at an EV of <$600m given their impressive science, good clinical data, strong partners and a reasonably well funded balance sheet with a quarter of the market cap cash.