Dear Readers,
This article will introduce three more Innovator CAR-T therapy companies: TCRR, CRSP, CRBU!
Let’s dig in:
10. TCR² Therapeutics (NASDAQ:TCRR, M.Cap: US$656m)
Founded in 2015 at MPM Capitals by Dr. Patrick Baeuerle, a world-renowned immunologist, TCR² is a clinical-stage immunotherapy company. They have propriety T cell Receptor Fusion Construct T cells (TRuC-T cells). By utilising the unique characteristics of TRuC-T cells they want to build potential cellular therapy for patients suffering from a wide range of solid tumors and hematologic cancers.
Advancements
1. TRuC-T cells
TRuC construct integrates into the TCR replacing the native CD3ε subunit
TRuC T cells have an intact TCR complex which has been effectively reprogrammed to recognise tumor surface antigens by fusing an antibody-based binding domain to TCR subunits.

Upon antigen engagement, TRuC-T cells harness the full internal signaling power of the complete TCR to produce a more powerful yet controlled T cell response against cancer. TRuC-T cells can recruit co-activating receptors (i.e. costimulatory molecules) similar to the natural TCR. This strategy differs from that of traditional CARs design which utilise only one of the six TCR subunits.
Conjugating the tumor antigen binder to the TCR complex further eliminates the need for HLA matching. TRuC-T cells seems to efficiently target solid tumors which often down-regulate HLAs thus preventing T cell detection.
TRuC-T cells demonstrate potent anti-tumor activity in both liquid and solid tumor xenograft models. They kill tumor cells as potently as second-generation CAR-T cells, but at a significantly lower cytokine release.
Enhancing the TRuCs platform
Enhancing with PD-1 Switch, IL-15, and dual TRuCs that co-express a PD-1:CD28 switch (TC-510, gavo-cel).
Dual TRuCs will have, enhanced early TCR downstream signaling, significantly increased proliferation, prevented exhaustion upon repeated antigen stimulation, augmented production of growth and effector cytokines, enhances efficacy of gavo-cel against PD-L1 overexpressing tumor.
Allogenic TRuC platform
Allogeneic TRuCs requires additional integration of non-alloreactive TCRαβ subunits to reduce GvHD risk and maintain the full functionality of the TCR
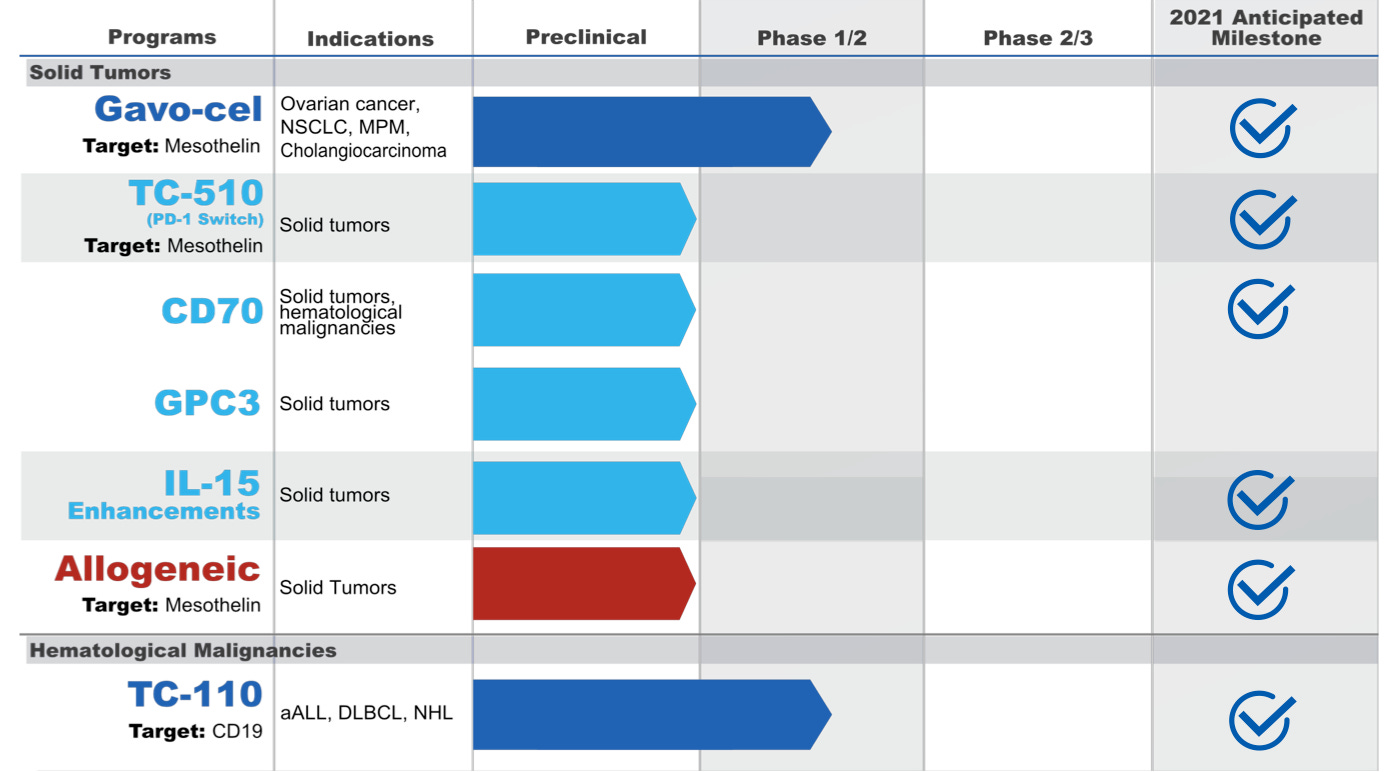
Product Pipeline
TC-210 (Gavo-cel): is a novel cell therapy that consists of autologous genetically engineered T cells expressing a single-domain antibody that recognizes human mesothelin, fused to the CD3-epsilon subunit which, upon expression, is incorporated into the endogenous TCR complex.
TC-210 therapy is to target advanced mesothelin-expressing solid tumors like non-small cell lung cancer, ovarian cancer, malignant pleural/peritoneal mesothelioma and cholangiocarcinoma. Mesothelin Solid Tumors Represent a Significant Market, with currently up to 80,000 patients in the US alone and expansion opportunity of 163,000 patients.
Mesothelin is expressed in mesothelial cells. It is highly expressed in a wide range of solid tumors and plays an active role in both malignant transformation and tumor aggressiveness. Since it is overexpressed in several cancers and is immunogenic, the protein is exploited as tumor marker or as the antigenic target of a therapeutic cancer vaccine.
Phase1/2 clinical studies are undergoing to determine the safety, recommended dose and then in Ph2 studies to test the efficacy (response rate) in solid tumors with or without enhancement therapy (checkpoint inhibitor: PD1); NCT03907852
Takeaways from the first cohort of patients treated with first suboptimal dose of TC-210 patients in the Phase 1/2 clinical trial: 100% Disease Control Rate, 38% ORR without lymphodepletion (LD) and 50% ORR with LD, 1 ovarian cancer and 2 (1 confirmed and 1 unconfirmed) mesothelioma RECIST partial responses (PR) were observed. Additionally, TC-210 was observed to have a manageable safety profile, with 2 CRS, no neurotoxicity or on-tumor, off-target toxicities.
TC-110: novel cell therapy that consists of autologous genetically engineered T cells expressing a single-domain antibody that recognizes human CD19, fused to the CD3-epsilon subunit which, upon expression, is incorporated into the endogenous TCR complex. Market size: up to ~14,000 patients in TC-110’s target indications.
Phase 1/2 open-label study is ongoing to evaluate the safety of TC-110 T cells in patients with aggressive NHL (DLBCL, PMBCL, TFL), high-risk indolent NHL (including MCL), or adult ALL; NCT04323657
Partners
Partnered with Cell and Gene Therapy Catapult UK in 2018 to build dedicated cGMP manufacturing suite then in 2020 in partnership with ElevateBio secured TC-210 clinical manufacturing capacity in US. ElevateBio is an innovative organization that has significant expertise in the cell and gene therapy field.
Key management
Dr. Patrick A. Baeuerle co-founder of TCR² Therapeutics, also co-founded other oncology portfolio companies Harpoon Therapeutics, iOmx Therapeutics, Maverick Therapeutics (acquired by Millenium Pharmaceuticals), Werewolf Therapeutics and Cullinan Oncology at MPM Capitals.
Dr. Baeuerle did groundbreaking research on transcription factor NF-kappaB and developed first FDA approved bispecific immunotherapy BLINCYTO, bispecific CD19-directed CD3 T cell engager antibody construct.
Financials
Net Cash of US$ 290m as of 2Q21 with a quarterly cash burn of around US$ 24m which gives them a runway through 2023.
11. CRISPR Therapeutics (NASDAQ: CRSP, M.Cap: US$ 8.9b)
It is a leading gene editing company, founded in 2012 by co-inventor of CRISPR-Cas9, Dr. Emmanuelle Charpentier. In 2016, the company went public on NASDAQ. Company is focused on developing transformative gene-based medicines for serious diseases like hemoglobinopathies, oncology, regenerative medicine and rare diseases using its proprietary CRISPR/Cas9 platform. CRISPR/Cas9 is a revolutionary gene editing technology that allows for precise, directed changes (disrupt, delete, correct or insert) to genomic DNA. CRISPR Therapeutics is developing it’s own portfolio of allogenic CAR-T products with an aim to overcome the primary challenges of the current generation of CAR-T therapies.
Advancements
CRISPR/Cas9 gene-editing is employed to eliminate or insert genes to create a new class of Allogenic CAR-T construct. Three basic steps are followed: 1. Insert the CAR construct precisely into the TCR alpha constant (TRAC) locus, 2. Eliminate the TCR with high efficiency and 3. β2M knock-out to eliminate Class I major histocompatibility complex (MHC I) expression.
Precise insertion of CAR construct helps to design much safer and more consistent product in contrast with the current generation of CAR-T products which uses randomly-integrating viruses (lentivirus or retrovirus) to deliver the CAR construct to the DNA of T cells.
Eliminating TCR with high efficiency and MHC I expressed on the surface of CAR-T product candidates, reduces the risk of GvHD and rejection of the CAR-T product by the patient’s own T cells, respectively. This strategy helps to reduce off-target effects, avoid need for more toxic lymphodepletion regimens, improve CAR-T cell persistence and increase the chance for durable remissions.
Allogeneic CAR-T enables simplified trial design: short screening timeframe, no apheresis, no bridging chemotherapy, and on-site availability of CAR-T cell product.
CRISPR gene editing facilitates consistent, multiplex editing to produce allogeneic cell therapies with enhanced immune cell performance and speed the discovery and generation of novel therapeutic candidates. They have seen that multiplexed, single-shot 6x knock-out plus CAR insertion performed at high efficiency: no viability decrease, no cytokine-independent growth and robust target-specific cytotoxicity.
Product Pipeline

CTX110: allogeneic CAR-T targeting CD19 for the treatment of CD19+ malignancies
Clinical Trial: CARBON, single-arm study evaluating the safety and efficacy of CTX110 in R/R non-Hodgkin lymphoma, as evidenced by 2+ lines of prior therapy; NCT04035434
Data as of Sep 2020: 12 patients were recruited for the clinical trial
CAR-T cells detected at multiple time points in all patients in DL2-4, with consistent peak expansion of CTX110 in the peripheral blood seen around 1-2 weeks post infusion and CTX110 detected out as late as 180 days after administration
Acceptable Safety Profile was observed with CTX110 at DL3 and Below. No GvHD despite all patients with ≤3/12 HLA match to CTX110 donors, No CRS or ICANS above Grade 2, No infusion reactions, 4 SAEs following CTX110 infusion not related to disease progression among 3 treated patients: ICANS (n=1), CRS (n=1), periorbital cellulitis (n=1), febrile neutropenia (n=1).
CTX120: allogeneic CAR-T targeting BCMA for the treatment of multiple myeloma
CTX120 has been granted Orphan Drug designation from the FDA. Ongoing Phase 1 clinical trial assessing the safety and efficacy of several dose levels in R/R MM.
CTX130: allogeneic CAR-T targeting CD70 antigen for the treatment of both hematologic malignancies and solid tumors (like renal cell carcinoma).
Received Orphan Drug Designation (ODD) from the U.S. FDA for CTX130 for the treatment of T-cell lymphoma. Ongoing two independent Phase 1 clinical trials assessing the safety and efficacy of several dose levels of CTX130, for the treatment of both solid tumors and certain hematologic malignancies.

Partners
In August 2016 the company started to operate Casebia Therapeutics, as a joint venture with Bayer. In 2019, Casebia Therapeutics came directly under the control of CRISPR Therapeutics.
Recent collaborations with Nkarta Therapeutics to co-develop and co-commercialise three gene-edited, donor-derived NK therapies and with Capsida Therapeutics to advance gene-edited therapies for familial amyotrophic lateral sclerosis and Friedreich’s ataxia.
Other collaborations include Bayer, Vertex Pharmaceuticals and ViaCyte, Inc.
Key management
Dr. Emmanuelle Charpentier, scientific founder, co-invented CRISPR/Cas9 gene editing and is the co-recipient of the Nobel prize in Chemistry for 2020.
Financials
The company has substantial cash reserves (Net cash of US$ 2.4b), which provides them adequate runway for many years.
12. Caribou Bioscience (NASDAQ: CRBU, M.Cap: US$ 1.6b)-New IPO
Caribou is a leading clinical-stage CRISPR genome editing biopharmaceutical company, founded by CRISPR genome editing pioneers, Dr. Rachel E. Haurwitz, Dr. Jennifer Doudna, Dr. Martin Jinek in 2011. On Jul 23, 2021, the company went public on NASDAQ with US$ 16 stock price. Company aims to leverage on breakthrough CRISPR gene editing to develop persistent allogenic CAR-T and CAR-NK cell therapies for a range of tumor types.
Advancements
Cas12a CRISPR hybrid RNA-DNA (chRDNA) Guide technology Platform: next-generation chRDNA enables multiple genome edits, including multiplex gene insertions, with a high degree of specificity and lower levels of off-target editing than first generation CRISPR-Cas9. Caribou owns >45 issued U.S. patents including 7 U.S. patents covering chRDNA technology.

Next Gen chRDNA guide technology: Source: Molecular Cell Article Cas12a chRDNAs mediate ~60-75% insertion rates in primary T cells and also maintains genomic integrity with reduced translocations (0.1%, compared to 3.5% translocation with standard CRISPR delivery technology). This is crucial as translocation of chromosomes can lead to cell dysfunction or formation of oncogenes.
Company believes that persistence is the key to unlocking the full potential of allogeneic cell therapies and used different strategies to innovate CAR-T design to deliver improved allogenic CAR-T persistence in patients
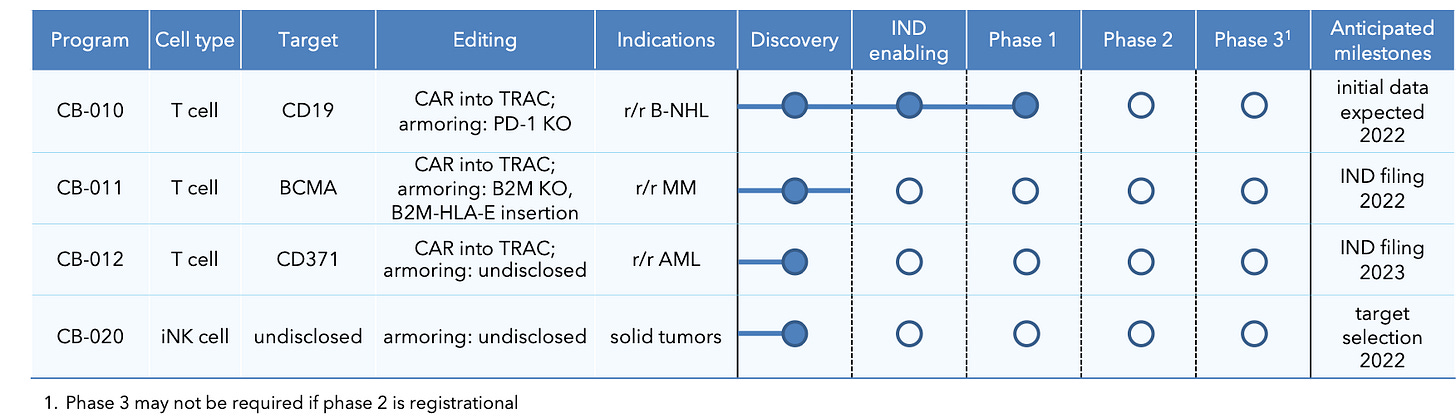
Product Pipeline
CB-010: Allogeneic anti-CD19 CAR-T with PD-1 knockout, first clinical stage
The PD-1 knockout is designed to prevent premature CAR-T cell exhaustion and thus to maintain high antitumor activity for a longer duration.
Clinical Trial: Ongoing Phase 1 open-label, multicenter clinical trial ongoing (ANTLER) to evaluate CB-010 in adults with R/R B cell non-Hodgkin lymphoma; NCT04637763.
CB-011: Allogenic anti-BCMA (uses proprietary, potent, humanized anti-BCMA CAR) CAR-T with immune cloaking to prevent rapid immune rejection of the CAR-T. This is achieved by B2M KO (removes all endogenous HLA class I presentation to prevent T cell-mediated rejection) + B2M–HLA-E–peptide fusion insertion (prevents NK cell-mediated rejection) using Cas12a chRDNA editing. IND filing planned in 2022.
CB-012: Allogeneic anti-CD371 CAR-T in AML (CD137 not expressed on HSCs, king it a compelling target). Cas12a chRDNA technology is used to armor CB-012 to improve the persistence of it’s antitumor activity. IND filing planned in 2023.
CB-020: Allogeneic iNK cell therapy for solid tumors. Discussed in detail in CAR-NK series (stay tuned!)
Partners
AbbVie: entered into a collaboration and license agreement in Jan 2021, for the research and development of CAR-T cell products. Under the multi-year agreement, AbbVie will use Caribou’s next-generation Cas12a CRISPR hybrid RNA-DNA (chRDNA) genome editing and cell therapy technologies to research and develop two new CAR-T cell therapies directed to targets specified by AbbVie.
The Leukemia & Lymphoma Society: In March 2021, Caribou announced a $115M Series C financing. As a part of the capital raise, Caribou received a strategic investment from The Leukemia & Lymphoma Society Therapy Acceleration Program® directed toward advancing CB-010, the company’s allogeneic CAR-T cell therapy product candidate for the treatment of relapsed or refractory B cell non-Hodgkin lymphoma.
Caribou licenses it’s CRISPR-Cas9 IP and certain of its own Cas9 IP to other companies for use in multiple market sectors including but not limited to research tools, transgenic research animals, internal research, diagnostics, and industrial biotechnology.
Key management
Dr. Rachel E. Haurwitz: Co-Founder, CEO, Pres & Director
Dr. Jennifer Doudna (Nobel Prize winner), Dr. Martin Jinek: Co-Founders and Scientific Advisors
Financials
In July 2021, Caribou completed its IPO, raising US$ 349.6 M in gross proceeds. Caribou finished the second quarter of 2021 with cash and cash equivalents of US$ 129.5 million (excludes IPO cash). While their cash burn should go up, the current balance sheet should provide a runway for the foreseeable future.
Supplementary Info:
Clinical trials based on the use of CRISPR-Cas9 technology registered in ClinicalTrials.gov (June 2021) excluding the clinical trials carried out by Crisper Therapeutics (4 ongoing Ph1/2 clinical trials) and Caribou (1 ongoing Ph1 trial):