CAR-Macrophage: A New Candidate For Treatment of Solid Tumors
Carisma Therapeutics, Myeloid Therapeutics, Thunder Biotech
Dear Readers,
Welcome to SciX newsletter. If you are new to SciX and want to get a view of what to expect in the articles, please click here.
In this article, we will explore a recent member of the CAR world: engineered monocytes/macrophages (CAR-M). We will discuss 1) the unique properties of these cells which makes them an attractive candidate of cellular medicine 2) explore biotech companies developing CAR-Mo/Mac (Carisma Therapeutics, Myeloid Therapeutics, Thunder Biotech) and compare their assets, 3) learn about the ongoing clinical trials and interim findings, 4) discuss the limitations and finally 5) evaluate whether this sector is worth investing.
Why we need new CARs ?
Advances in CAR engineering has opened up new vistas for cellular therapy. CAR-T therapy has shown promise in haematological malignancies, but its application to solid tumors has been challenging. Solid tumors are tricky to treat with CAR-T, this is partly due to the fact that T cells can’t easily find, penetrate and survive in the tumour microenvironment (TME). To overcome these limitations, immune cells of innate (NK, Mac, DC, γδ-T), subset of lymphocytes that bridge the innate and adaptive immune systems (iNKT) and specialised cells of acquired immune system (Treg) are being explored as new candidates for CAR cellular medicine platform. This article will focus on CAR-M, subsequent articles will analyse other new candidates of CAR world. Stay tuned and subscribe to SciX newsletter.
Subscribers receive every post of SciX newsletter directly in their inbox and an opportunity to ask questions and submit suggestions!
In the previous article, we took a deep dive into CAR-NK and CAR-iNKT assets which are showing promising results in multiple ongoing Phase 1 clinical trials. They are better than CAR-T cells for two major reasons. First there are no serious side effects associated with CAR-NK therapy, like CRS and neurotoxicities. Second, it can be developed as an allogeneic therapy with no concern about GvHD.
Why are Myeloid cells getting CAR?
Myeloid cells, including Monocytes and Macrophages are central regulators of the innate immune system, body’s first line of defence and plays a crucial role in choreographing downstream immune responses. Myeloid cells can also help the immune system guard against cancer.
They express a wide range of innate immune sensors such as pattern-recognition receptors-Toll-like receptors, C-type lectin receptors, cGAS-STING, RIG-1 and co-stimulatory molecules like CD40, MyD88.
They are naturally recruited to solid tumors and can survive within the tumor microenvironment (TME). They represent the largest population of immune infiltrates in all tumors, in some cases contributing up to 75% of the tumor mass.
Macrophages and Monocytes also have the potential to phagocytose tumor cells, activate the TME, and prime a broad anti-tumor adaptive immune response via T cell recruitment and activation.
Macrophages are functionally plastic cells capable of adopting different phenotypes. In response to environmental cues, macrophages show spectrum of polarised states which range from the pro-inflammatory/anti-tumor M1 phenotype to anti-inflammatory/pro-tumor M2 phenotype. This dual behaviour of macrophages has been a double-edged sword in immuno-oncology.
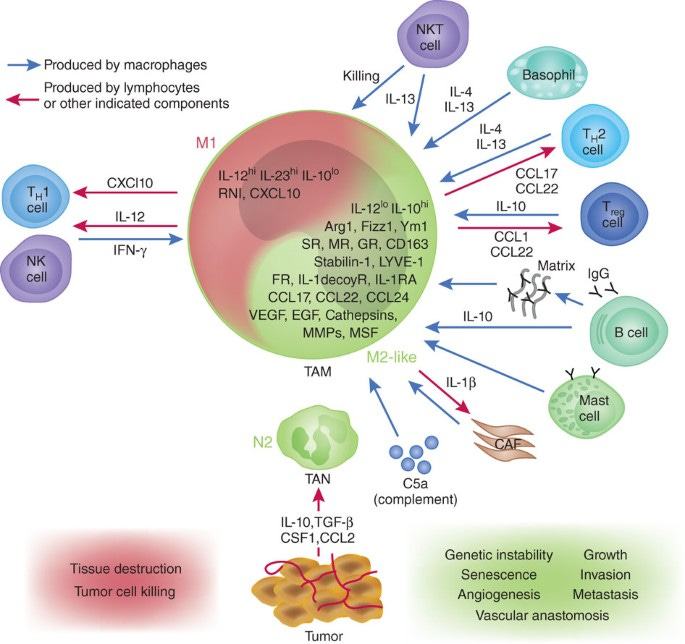
Macrophages play critical and complex role in tumor biology.
Ad5F35 solves a key challenge of engineering Macrophages
It has always been a challenge to genetically engineer macrophages. However, it is now possible with chimeric adenovirus vector, Ad5F35. Ad5F35 can mediate gene transfer into human macrophages at higher efficiency and most importantly the transduced macrophages display a pro-inflammatory/anti-tumor phenotype and also resist polarisation to anti-inflammatory/pro-tumour phenotype.
Other recently discovered delivery methods to engineer macrophages are Vpx-LV (Virus-like particles-Lentiviral Vector), modified mRNA (for transient macrophage engineering), LNP mRNA delivery (Lentiviral NanoParticle) and CRISPR/Cas9.
Gene transfer using viral transfection may induce insertion mutations and have an uncontrollable effect on treatment. Such issues can be resolved with CRISPR/Cas9 gene editing, which can also reduce the manufacturing time. Other improved gene-editing and delivery methods are being tested and ongoing studies will tell us more about their efficacy and safety.
Biotech Companies developing CAR-M
1. Carisma Therapeutics Inc. (NASDAQ: CARM)
Carisma Therapeutics Inc. is a privately held biopharmaceutical company founded in 2016 and headquartered in Philadelphia, PA. The company recently announced their merger with Sesen Bio (Nasdaq: SESN) to advance Carisma’s cell therapy platform, in particular, its CAR-M therapies. The merger is still pending and both the companies on Dec 29, 2022 announced the revised terms of the merger. According to amended merger agreement, Sesen Bio Stockholders expected to receive approximately $70M special cash dividend, an increase from up to $25M previously announced. The merger and related financing are expected to close in the first quarter of 2023, subject to approval by Sesen Bio stockholders and other customary closing conditions. After the completion of merger company will be listed on Nasdaq as CARM.
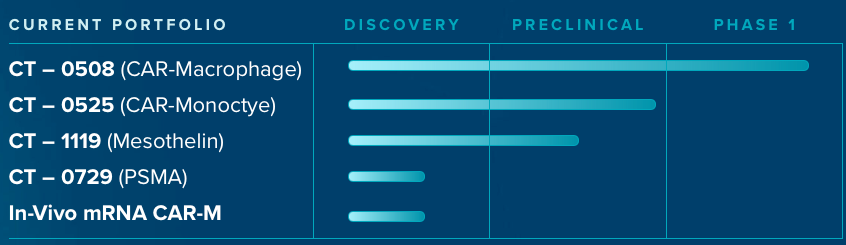
Company’s first asset, autologous CAR-M (CT-0508) is based on proprietary technologies that were developed by leading scientists at the University of Pennsylvania. CT-0508 is currently in Phase 1 clinical trial and on Sept, 2021 received Fast Track Designation from FDA. Carisma is also developing a CAR-Monocyte therapy with a 1 day processing time and plans to file an IND in 2023. Besides CT-0508, company’s assets are either in preclinical or discovery phase (see below pipeline chart).
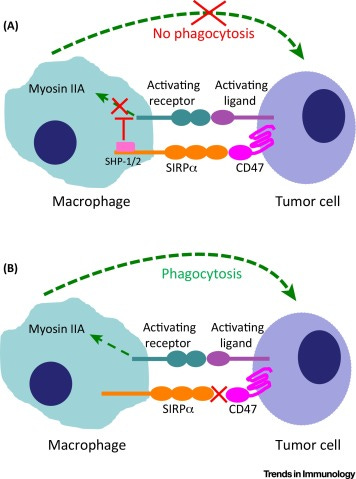
Besides, company is also designing second generation CAR-Ms with additional innate immune activation receptor (CD40, MyD88). This second signalling domain can improve the killing/phagocytosis potential of CAR-Ms, enhance anti-tumor cytokine/chemokine release and more potent M1 phenotype. Additionally, company is also enhancing CAR-M potential through gene editing SIRP-alpha (macrophage checkpoint that inhibits phagocytosis) via CRISPR/Cas9.
Combining additional activation domains to CAR-M is a potential attempt and furthermore by blocking the "don't eat me" signal CD47-SIRPα may enhance CAR-M's phagocytosis of targeted cancer cells. The impact of SIRPα–CD47 blockade may be further improved by relieving additional constraints on phagocytosis.
Company recently also entered into a strategic partnership with Moderna (Nasdaq: MRNA) for the discovery, development and commercialisation of in vivo CAR-M therapies for up to 12 targets for the treatment of cancer. Moderna’s mRNA and lipid nanoparticle delivery technology will be a potentially game-changing opportunity for engineering macrophages for in vivo therapies.
CT-0508
Autologous CAR-M expressing humanised anti-HER2 ScFv developed for the treatment of heavily treated metastatic HER2 over-expressing solid tumors (like breast cancer, oesophageal, colangiocarcinoma, salivary carcinoma, and ovarian cancer).
Interim data from an ongoing Phase 1 study (NCT04660929) supports safe profile and mechanism of action. CT-0508 anti-HER2-CAR-M are skewed towards M1 phenotype, i.e., anti-tumor phenotype.
Till now, company has released data of 7 patients that were administered 1.43 billion (median dosing) anti-HER2-expressing CAR-M cells. 5/7 patients received D1/D3 and 2/7 received D1/D3/D5 infusion of CAR-Ms. Patients dis not required pre-conditioning chemotherapy treatment, which is required with CAR-T and CAR-NK cells. Preparative chemotherapy poses additional challenge to cancer patients and make them more vulnerable to future infections. The patients treated with CAR-M therapy were heavily pre-treated and with metastatic grade cancer.
No dose limiting toxicities, no severe CRS, ICANS or any major organ toxicity was observed in all 7 patients treated with CT-0508. Transient and low grade fever was observed in 5/7 patients which was resolved in 48h.
Tumors of CT-0508 treated patients demonstrated increased infiltration of CD8 T cells and M1 macrophages suggesting induction of anti-tumor immunity. In some patients, a fraction of infiltrated T cells demonstrated exhaustive phenotype and also CT-0508 CAR-M displayed over-expression of PDL1. Company is now pursuing this data by planning to combine CT-0508 with PD1 inhibitor (Keytruda) in collaboration with Merck.
Miltenyi Biotech and Novartis are company’s manufacturing partners. Their manufacturing process is mostly automatic and vein to vein time is about 3 weeks. No chemotherapy conditioning is required for CAR-M therapy.
2. Myeloid Therapeutics (Private, $50M Series A funding)
Founded in 2019 by a group of world-leading scientists and experts of oncology, immunology, mRNA biology, cell therapy, synthetic biology, and gene delivery.
Company’s proprietary ATAK platform which stands for Activate, Target, Attack & Kill, utilises the combination of tumor-associated antigen recognition domains and intracellular signalling domains from innate immune receptors (such as FcR, TLR and cytokine receptors) to control and program myeloid cells to recognise cancer and elicit a broad and controllable immune response.
With ATAK platform, by associating different domains, company has designed structure of CAR which is most suitable for myeloid cells to enhance the phagocytic effect of CAR-M on cancer cells.
In addition, company has streamlined its manufacturing for its ATAK cell therapy candidates through a rapid, single-day cell process, which provides significant advantages to the patients.
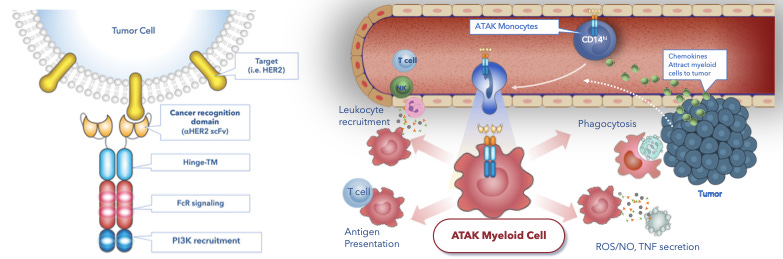
Click the link, if interested to learn more about CARs that trigger phagocytosis.
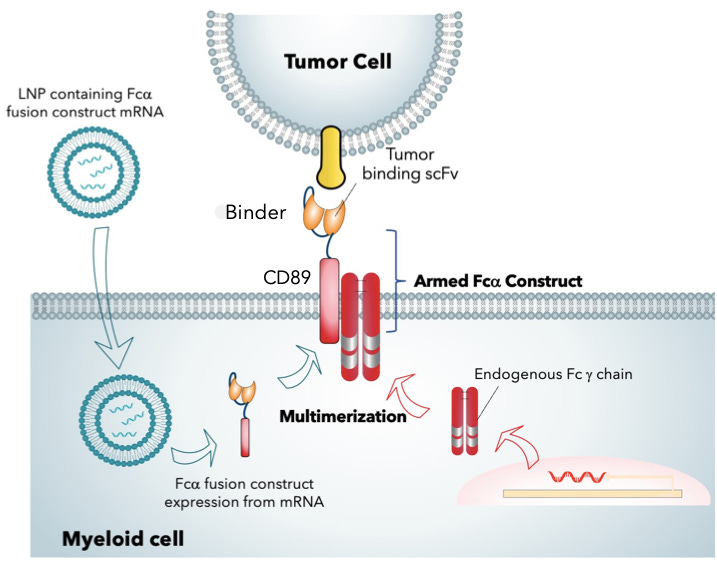
Myeloid Tx has developed a in vivo myeloid engineering platform which will enable first-ever delivery of mRNA-encoded CARs directly to Humans. Unlike other CARs, the construct is engineered by fusing a tumor recognition scFv with the alpha chain of human Fc receptors (CD89). Thereafter, intravenous infusion of lipid nanoparticle (LNP) encapsulating mRNA encoding the FcaR fusion protein results in uptake of LNPs and expression of chimeric receptor fusion protein in myeloid cells. The efficacy of LNP mRNA delivery to program myeloid cells has been tested in immunodeficient xenograft models of hepatocellular and triple negative breast cancer and also in B16/10 syngeneic melanoma model.
On May 19, 2022, company entered into agreement with Acuitas Therapeutics, a global leader in developing LNP delivery systems that enables mRNA-based therapeutics for LNP System to develop next-generation immunotherapies for cancer.
Besides company is also working on refining gene-editing and delivery using proprietary RNA-based, retrotransposon mediated gene-insertion technology, (RetroT). This breakthrough technology offers the potential to deliver gene-sized pieces of DNA into the genome and thus significantly expanding the type and scope of genetic errors that can be reversed in situ. This is not possible with existing gene editing technologies. In March 2022, company entered an exclusive research collaboration and option agreement with Prime Medicine to develop and accelerate RetroT.
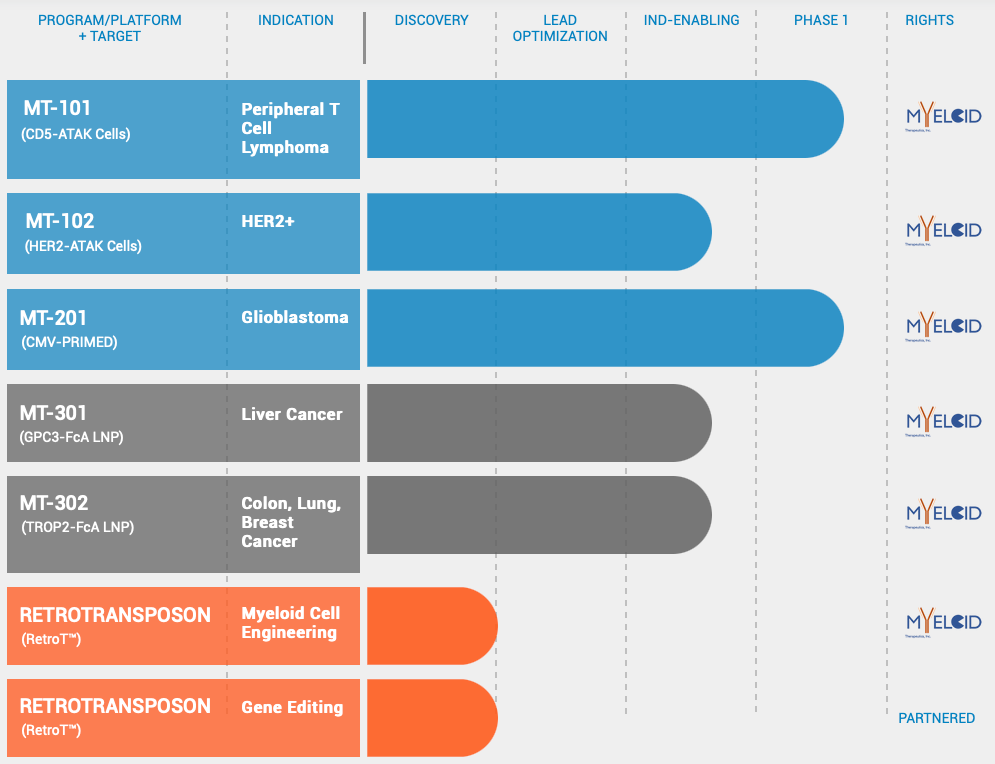
Pipeline
MT-101 (CD5-ATAK Monocytes)
MT-101 is mRNA engineered CAR Monocyte, created from the proprietary ATAK platform. It is being developed for the treatment of relapsed and refractory CD5-expressing T cell lymphomas. Refractory PTCL an aggressive form of non-Hodgkin lymphoma is a lethal disease with limited treatment options, that has not benefited from innovations in cell therapy. MT-101 targets CD5, a surface receptor present on greater than 75% of PTCL. It is engineered with a novel CAR design that triggers innate potential of monocytes to kill tumor cells and orchestrate “cold” TME to immune-rich “hot” TME.
MT-101 was granted Fast Track Designation by FDA on Oct 28, 2022.
MT-101 can be manufactured in 1 day with vein-to-vein time of only 8 days thus can be delivered expeditiously to the clinic.
IMAGINE, a Phase 1/2 trial (NCT05138458) assessing safety, tolerability, and efficacy of MT-101 in this indication is open for enrolment and the initial data is very encouraging.
MT-102 is being developed for individuals with HER2 expressing cancers. In vivo studies show MT-102 has eradicated HER2+ tumors. MT-201, glioblastoma program is now being advanced to clinical stage in partnership with Duke University after showing potent anti-tumor activity in animal cancer models. MT-301 and MT-302, two in vivo programming candidates against validated cancer targets (GPC3 and TROP2, respectively) are being developed utilising Acuitas’ LNP deliver technology.
Company has raised $50M funding in over 1 Series A round on Jan 6, 2021. Well known biotechnology investors like Newpath Management, Hatteras Venture Partners, Alexandria Venture Investments and 8VC are funding Myeloid Therapeutics.
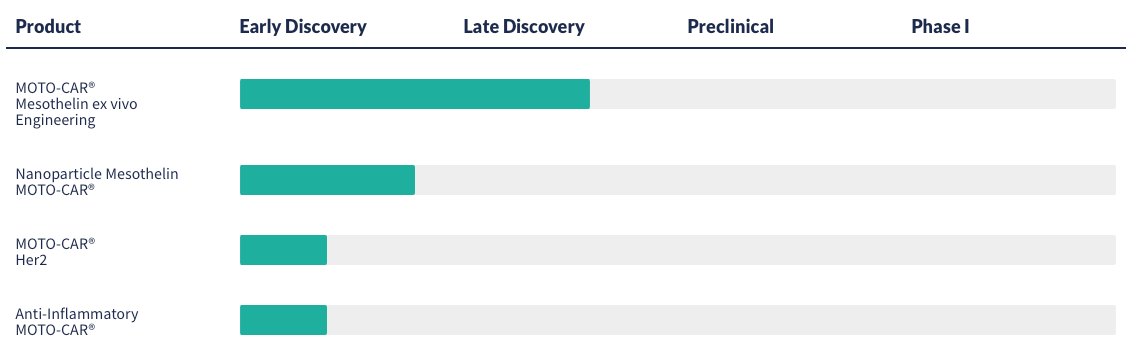
3. Thunder Biotech (Private, $24.4M in funding over 5 rounds)
Private biotech company utilising advanced MOTO-CAR immunotherapy platform to generate CAR-M therapies for solid tumors.
MOTO-CARs are macrophages genetically engineered with receptors that bind to tumor neoantigens. Thunder Biotech is also targeting the well established solid tumor markers, like Mesothelin, HPRT and HER2. Company’s assets are in discovery phase.
Thunder Biotech has raised a total of $24.4M in funding over 5 rounds. Their latest funding was raised on Oct 13, 2022 from a Debt Financing round.
Current Status of CAR-M Studies
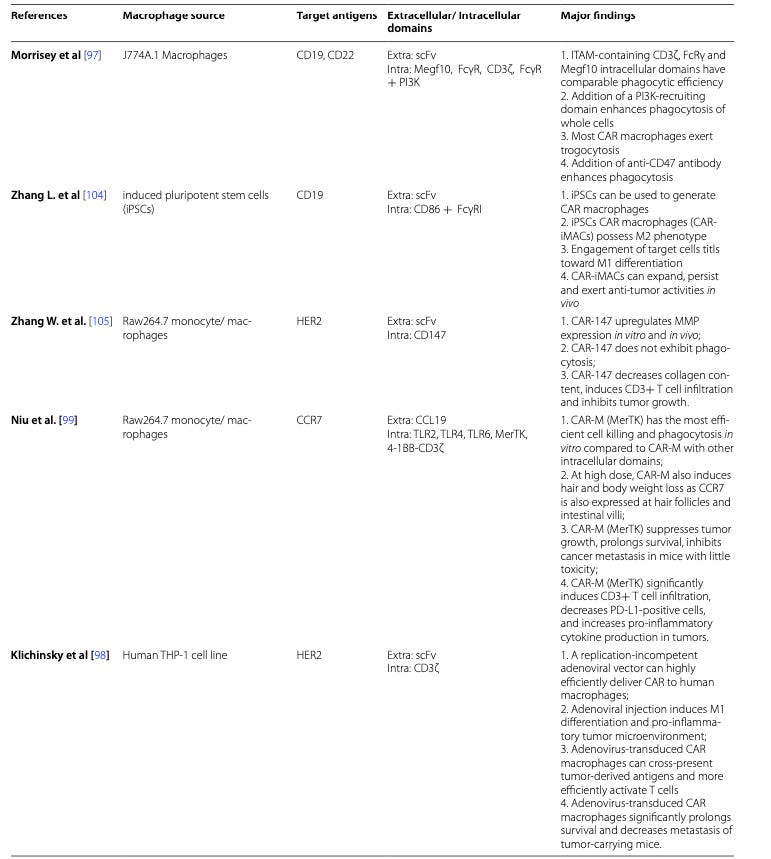
Summary of Clinical Findings
Preclinical studies and ongoing Phase 1/2 clinical studies (NCT04660929; NCT05138458) have demonstrated some remarkable properties of CAR-M cells over CAR-T with respect to solid tumors. In contrast to CAR-T cellular therapy:
1. CAR-M cells can readily penetrate tumor microenvironment, orchestrate secondary immune response which coverts a “cold” (immune deprived) tumor to a “hot” (immune-rich) one,
2. CAR-M infiltration to TME can reduce the ratio of TAM and affect the phenotype of TAMs to an inflammatory anti-tumor type
3. In addition to the phagocytosis of tumor cells, CAR-M is also able to promote the ability of antigen-presentation and enhance the cytotoxic effects of T cells.
4. No DLC, ICANS, Grade 3/4 CRS response was observed with CAR-M.
Below table compares two new CAR members-CAR-NK and CAR-M to FDA approved CAR-Ts.

CAR-M therapy is a beacon of hope from solid tumors. For patients with heavy burden of solid tumors, it’s worth to try to combine CAR-M with CAR-T. CAR-M can repress the release of cytokines throughout the treatment process, which may reduce the risk of CRS and neurotoxicity from CAR-T therapy. Also, combining CAR-M therapy with other immunotherapy is also a potential attempt.
Limitations of CAR-M
Although CAR-M has great potential to become a powerful cancer immunotherapy, many problems need to be overcome in order to achieve devised results which can translated to clinics. Some of the major hurdles which need attention are as follows:
Macrophages do not proliferate either in vitro or after injection in vivo and furthermore, patients can accept macrophages only in limited amounts, which may affect the effectiveness of treatment. Current ongoing trials are injecting around billion CAR-M cells, which so far have not resulted in cytotoxicity and also demonstrated infiltration in tumor microenvironment. However, it will be crucial to see the tumor regression data and the phenotype of CAR-M overtime in dynamic tumor microenvironment.
Migration characteristics of macrophages in vivo: After injection, exogenous macrophages pass through the lung, and then most of them remain in the liver, which is not conducive to the treatment of cancer.
Dynamic tumor microenvironment: For anti-tumor activity it is pre-requisite for CAR-Ms to remain stable in classical, pro-inflammatory phenotype, however, TME cues promote pro-tumour TAM phenotype. Therefore, it will be crucial to observe how this dynamic situation plays out in long run.
Finally, due to the high heterogeneity of tumor cells, the expression of target antigens may not be sufficient.
Conclusion
In conclusion, CAR-M is still at its nascent stage with two clinical trial initiated and promising initial results reported from one clinical trial. Any of the serious limitations have yet to be unfolded. However, there are high hopes from this new candidate for treatment of solid tumors since tumor-associated macrophages are the major cell type and main immune regulator at TME, advances in CAR designing and research direction is to develop CAR-M not only as a phagocytic machinery but more importantly, an antigen presenter, TME modifier and immune stimulator to promote anticancer immunity.