Dear Readers,
Science Xplained (SciX) is back!!
If you are new to SciX newsletter and want to get a view of what to expect in the articles, please click here.
In the past posts, we unraveled the scientific world of cell based therapies by taking a deep dive into 16 CAR-T/TCR companies. Since my last post a lot has changed in the world. Therefore, this article is dedicated to get an overview of the major events of CAR-T world, understand the current landscape of CAR-T therapies and to re-evaluate whether CAR biotech sector still holds any promise as an investment ?

Major events of CAR-T space
Long-lasting remissions in r/r ALL patients
CAR T therapy is expensive, risky and technically demanding. However, it remains a last resort for cancer patients, to be used when all other treatments have failed. The long term follow up studies from children with relapsed acute lymphoblastic leukemia (ALL) who had been treated with CAR-T cells as part of the first clinical trials was recently published. The study reported that approximately 60% of children treated with autologous CD19-CAR T followed by potentially curative stem-cell transplant were still alive 5 years later without their cancer coming back or the children experiencing any disease-related problems. CD19-targeted CAR-T cells have induced complete remissions of disease in up to 90% of patients with relapsed or refractory B-cell-ALL, who have an expected complete response rate of 30% in response to chemotherapy.
Decade-Long remissions in r/r CLL patients
The potential impact of CAR-T is tremendous. Two of the first few people with chronic lymphocytic leukaemia (CLL) treated with CD19-CAR-T cancer therapy are still in remission 12 years on. Carl June and his team tracked the treatment long-term to understand as to what factors are important for lasting CAR-T-cell success. They found that the CAR-T cells persisted, but the characteristics of the population shifted over time. Soon after infusion of CAR-T, there was dominance of killer or cytotoxic CD8 T cells (a type of T cells that can identify and kill cancerous or viral infected cells). In one patient, gamma delta CAR-T cells emerged and expanded during the initial phase of treatment. Over the years, both the patients in long-term remission displayed emergence of highly active CD4 T cells with specific characteristics that displayed they would be capable of killing leukaemia cells. This study is a proof of concept about the safety of having long-term persistence of active and functional CAR-T cells in the body.
However, such durable remissions are observed in only about 25–35% of CAR-T cell recipients with CML. With refinement of CAR-T cells, this percentage has increased over the years but some of these initial successes still lead to relapse. The insights from the long-term follow up studies will help to further refine the development of CAR-T cells to generate potent, functional and long-lasting CAR-T therapies.
It has been an exciting time in immune-oncology research, particularly in CAR-T space, in market there are 12 FDA-approvals, 6 products and 2 targets, CD19 or B cell maturation antigen (BCMA) for lymphoma, leukemia and multiple myeloma. [CD19 is a marker of B cells and BCMA is primarily expressed on the surface of malignant multiple myeloma B-lineage cells, as well as late-stage B-cells and plasma cells]. In the near future, we hope to see similar results for glioblastoma, the most aggressive form of brain cancer, as well as other solid tumors.
Recent FDA approval
On February 2022, FDA approved CARVYKTI™ (ciltacabtagene autoleucel), Janssen’s first cell therapy, a BCMA-directed CAR-T for the treatment of patients with r/r Multiple Myeloma. In December 2017, Janssen entered into an exclusive worldwide license and collaboration agreement with Legend Biotech to develop and commercialise ciltacabtagene autoleucel.
In the pivotal CARTITUDE-1 study, where patients with r/rMM with one-time treatment with BMCA-CAR-T resulted in deep and durable responses, with 98% Overall Response Rate. Notably, 78% of the patients achieving this level of response experienced a stringent complete response.
Some good news from CAR-T research on solid tumors
There are several challenges to target solid tumors with CAR-T therapies, one major challenge is the lack of a tumor-specific biomarker.
Recently team of scientists from Penn University have discovered a biomarker for gastrointestinal cancer and neuroendocrine tumors, CDH17. CDH17 is a cell surface adhesion protein, it is present on both cancerous and normal cells but it is ‘masked’ in healthy tissues. In pre-clinical trials, CDH17-CAR-Ts killed both human and mouse tumor cells sparing normal cells, making CDH17 a safe tumor-specific biomarker. Australian based biotech company, Chimeric Therapeutics (CHM.AX) has in-licensed the CDH17-CAR-T cell therapy technology to develop 3rd generation (with both CD28 and 41BB co-stimulatory domains) CAR T cell therapy, CHM 2101 to test against neuroendocrine tumours, as well as gastrointestinal (GI) cancers such as colorectal, pancreatic and gastric cancer.
Another potential target for solid tumors is a novel oncofetal antigen Claudin 6 (CLDN6), a tumor-specific antigen widely expressed in various solid tumors such as ovarian cancer, sarcoma, testicular cancer, endometrial cancer and gastric cancer but silenced in healthy adult tissues. BioNTech (BNTX) has combined their CAR-T and FixVac platform technologies to design autologous CAR-T targeting CLDN6 antigen which is consecutively enhanced by a CAR-T Cell Amplifying RNA Vaccine (CARVac). The combined treatment is dubbed as BNT-211. This is first in kind approach where RNA vaccine is combined with CAR-T to boost persistence and functionality of CAR-T cells. Findings from a pre-clinical study showed improved engraftment of CAR-T cells and regression of large tumors in difficult-to-treat mouse models was achieved at sub-therapeutic CAR-T cell doses. Additionally, the preliminary results demonstrated an encouraging safety profile and anti-tumor activity in testicular cancer patients at the first evaluated dose levels of BNT211. These positive interim data has also helped BNT211 to receive Priority Medicine (PRIME) designation from EMA for enhanced regulatory support.
Another biomarker discovery is for Neuroblastoma (NB), an extremely rare and notoriously difficult-to-treat form of cancer that mainly affects children. To date, 6 NB tumor associated antigens (TAA), including GD2, CD171, GPC2, CD276 (B7-H3), ALK, and NCAM-1, are being developed as targets for CAR-T cell therapies. Only CAR-T cell therapies against GD2, L1-CAM, and CD276 (See Table below) have reached clinical trials and some, using CARs against GD2 and L1-CAM, have reported results. Recent studies indicate GPC2 as a potential candidate, it is significantly overexpressed in multiple pediatric cancers including NB, with low or undetectable expression in normal tissues. GPC2 is critical for growth and differentiation of neurons in the developing nervous system. NB is difficult to target, one reason being heterogenous TAA expression on tumor cells. To overcome this challenge, a bicistronic CAR (BiCisCAR) targeting both newly the identified biomarker GPC2 and CD276, was generated and optimised. CD276 is a checkpoint molecule involved in tumor immune evasion and metastasis and has substantiated its efficacy in early stage clinical trials.
It will be interesting to watch the clinical development of CHM 2101, BNT211 and BiCisCAR. This list is not exhaustive, due to limited space I’ve just picked the most interesting ones. Let me know by leaving a comment, if you are interested to learn more about TAA developments.

Current Landscape of CAR-T therapies
Strong pre-clinical or Phase 1 clinical validation of CAR-T therapies
Cellectis (CELLS) product candidate UCART123, an allogenic (off-the-shelf) TCRαβ deficient CAR-T cells targeting CD123 in AML, showed strong preclinical validation, effectively eliminating AML cells in vitro and in vivo with improvements in overall survival and minimal impact against normal hematopoietic progenitors. UCART123 also showed promising efficacy in specific killing of BPDCN cells in vitro and in xenograft (PDX) mouse models. UCART123 is currently being evaluated in the Phase 1 dose-escalation trial AMELI-01 in patients with relapsed or refractory AML.
Juno Therapeutics/BMY led Phase 1 clinical trial of MCARH109 (GPRC5D-CAR T) on patients with relapsed or therapy resistant multiple myeloma sparked remissions in 70.6% of the 17 patients in the trial. The 17 relapsed patients in the phase 1 trial had undergone a median of six prior treatments for myeloma, including several who had received previous generation of CAR T-cell therapies, which target a cell antigen known as BCMA. GPRC5D is G protein-coupled receptor, class C group 5 member D present mainly on Myeloma cells, this antigen is still a mystery as its role in normal cell life is unclear.
Preliminary efficacy data from Caribou Biosciences (CRBU) led ANTLER Phase 1 trial in r/r B-NHL with CB-010 (Allogeneic Anti-CD19 CAR-T with a PD1 KO using CRISPR-genome-editing) is promising. All six patients treated in this study showed 100% (6/6) complete response (CR) after treatment with single dose of CB-010, at 6 months assessment, 40% (2/5) showed CR. Initial ANTLER data are an important step toward validating Caribou’s chRDNA genome-editing platform, which has the potential to minimise unintended editing at off-target sites. Except CB-010, other CAR-T therapies of CRBU are in discovery phase.
TCR2 Therapeutics (TCRR) recently announced data of their Phase 1/2 clinical trial of GAVO-CEL (T cell receptor fusion construct directed against mesothelin) in 32 patients (including 23 mesothelioma, eight ovarian cancer and one cholangiocarcinoma). The ORR among patients who received gavo-cel following lymphodepletion chemotherapy was 22%. The Phase 2 portion of the study will be in combination with checkpoint inhibitors, and also they will retreat patients with additional doses of gavo-cel, potentially translating into even higher response rates and improved durability of benefit. A competitive Phase 1 clinical trial with MSLN-targeted CAR-T cells (ATA2271) in combination with anti-PD1 inhibitor in Patients With Mesothelioma has been initiated with Atara Biotherapeutics (ATRA). They have also initiated IND enabling studies of off-the-shelf, allogeneic EBV mesothelin CAR T cells (ATA3271).
Allogene Therapeutics (ALLO) pipeline includes CAR T candidates targeting CD19 (ALLO-501A, ALLO-647), BMCA (ALLO-715) and CD70 (ALLO-316). It is the only midscale company with four ongoing Phase 1 clinical trials. It will be interesting to watch this company closely.
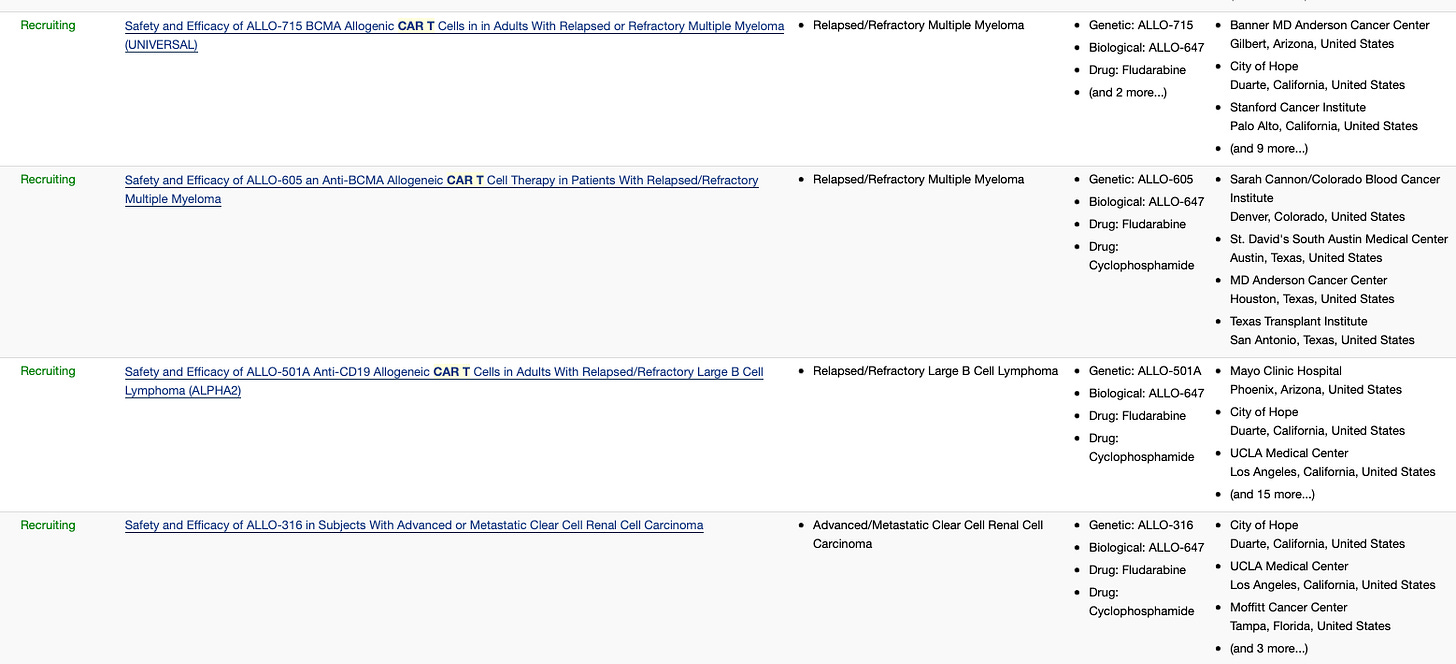
Recently, the FDA granted Regenerative Medicine Advanced Therapy (RMAT) designation to ALLO-501A in r/r LBCL.
Crisper Therapeutics (CRSP) recently was granted RMAT status for its CTX130 asset, an allogenic CAR-T therapy targeting CD70 for the treatment of Cutaneous T-Cell Lymphomas (CTCL). RMAT status is an important milestone, it recognises the transformative potential of cell therapy in patients with T-cell lymphomas. CTX130 is being investigated in two ongoing independent Phase 1 clinical trials that are designed to assess the safety and efficacy of several dose levels of CTX130 in adult patients. Crisper Therapeutics has established strategic partnerships with leading companies including Bayer, Vertex Pharmaceuticals (VRTX) and ViaCyte, Inc. Their another CAR-T asset, CTX110, allogenic CD19-CAR-T for relapsed/refractory non-Hodgkin lymphoma showed promising response with well tolerance in initial studies. Company is planning for registrational trial for CTX110. Proof-of-concept for allogeneic CAR-T achieved with CTX110 and CTX130. Across 4 clinical trials with CTX110 and CTX130, >100 patients dosed with CRISPR-edited CAR-T cells showed durable and promising efficacy.
Watch these two companies closely as Crisper and Vertex are planning to submit BLA/MAA filing in Q42022 of CTX001 (Exa-cel) for the treatment severe SCD and β-thalassemia, if approved it will be the first ever CRISPER-based medicine in market.
Gracell Biotechnologies (GRCL) announced the updated clinical data of BCMA/CD19 Dual-Targeting FasTCAR-T GC012F, for the treatment of R/R MM. 29 patients were treated in this trial, all patients 100% (29/29) achieved minimal residual disease (MRD) negativity. 75.9% (22/29) of all patients treated achieved MRD- sCR. Median duration of response at data cut off was 15.7 months (95% CI: 7.6-33.1). GC012F is the first BCMA/CD19 dual-targeting CAR-T in human trials for B-NHL and it holds orphan drug status, granted by FDA in Nov, 2021. Gracell is developing a rich clinical-stage pipeline of multiple autologous and allogeneic product candidates, with 4 of its assets in early clinical trials.
IN8Bio (INAB) shared the interim data from the Phase 1 clinical trial of allogeneic derived, gamma-delta T cells (INB-100). Three high-risk AML patients with complex cytogenetics have been treated to-date. The trial continues to show positive clinical trends with patients remaining alive and progression-free. No treatment emergent serious adverse events have been observed. Data update expected in Q4 2022. Another update comes from Phase 1 clinical trial of INB-200 in patients with newly diagnosed glioblastoma multiforme (GBM). INB-200 is a genetically engineered gamma-delta T cell product candidate that is designed to be chemotherapy resistant. As of June 3, 2022, six patients have been dosed with INB-200, 100% of dosed patients have exceeded both median and expected progression free survival. Two patients, to date, having exceeded their expected overall survival. Indeed promising data and proof-of-concept of company’s core Drug Resistant Immunotherapy (DRI) platform.
FDA lifts hold on CAR-T clinical trials
Allogene’s (ALLO) all five clinical trials of AlloCAR-T portfolio were put on hold in October 2021 by FDA based on the report of one patient with pretreated stage IV follicular lymphoma showing evidence of chromosomal abnormality after receiving ALLO-501A CAR-T cells as part of the ALPHA2 study. This clinical hold also affected Cellectis (CLLS) as they used TALEN gene editing in their CAR-T cells. FDA did an investigation and concluded that the chromosomal abnormality was unrelated to TALEN gene editing or Allogene’s manufacturing process, the clinical hold was lifted from all the five trials on Jan, 2022.
Celyad Oncology (CYAD) decided to voluntarily halt subject dosing and enrolment in Phase 1b trial of CYAD-101-002 for refractory metastatic colorectal cancer after reports of two fatalities with similar pulmonary findings. The trial was assessing the Celyad’s proprietary TCR Inhibitory molecule (TIM)-based allogenic NKG2D CAR-T cell with MSD’s anti-PD-1 therapy (pembrolizumab). On Aug 1, 2022, FDA lifted the clinical hold after the Company made changes to the eligibility criteria for the trial.
Celyad expertise is in non-gene edited (shRNA) technologies, CYAD-101 is currently their only clinical allogeneic CAR T co-expressing NKG2D and TIM. No dose-limiting toxicities were observed in 25 patients treated with CYAD-101 in the alloSHRINK Phase I trial.
Dropping clinical assets and Rebranding
Ziopharm Oncology (ZIOP) reshuffled and rebranded itself with dropping clinical assets (IL-12 clinical program), 15% staff reduction, delaying phase 1/2 clinical trial of T-cell receptor, new office location, new CEO to Alaunos Therapeutics (TCRT). Majority of their assets are in early stage development. They have started enrolling patients in Phase1/2 study of T-cell receptor against different solid cancer.
Bluebird Bio (BLUE) restructured its workforce by cutting 30% workforce and also deprioritised direct investments in reduced toxicity conditioning and cryopreserved apheresis. Company is prioritising to reduce operating cost and sharpen its focus on near-term catalysts, including anticipated FDA approvals for its gene therapies for beta-thalassemia and cerebral adrenoleukodystrophy in 2022.
As planned in November 2021, 2seventy bio (NASDAQ: TSVT) was spun out of bluebird bio. Together with BMS they have the FDA approval of ABECMA (idecabtagene vicleucel; ide-cel) treatment of r/r multiple myeloma. BMS reported total U.S. ABECMA second quarter revenues of USD 72 million, representing 29% growth over the prior quarter. The collaboration experienced increased ABECMA manufacturing costs driven primarily by higher than anticipated vector costs. 2seventy bio has plans to initiate clinical trial of ABECMA in newly diagnosed multiple myeloma patients.
CAR-T therapy for Heart failure
Group of scientists from Penn University have developed a therapeutic approach to generate transient CAR-T cells in vivo by delivering modified messenger RNA (mRNA) in T cell–targeted lipid nanoparticles (LNPs); CD5-targeted LNPs to generate anti-fibrotic CAR-T cells. These in vivo–reprogrammed CAR-T cells were generated to target the fibrotic area in the heart and restore the damaged heart. Findings from mouse model with heart failure showed that treatment with anti-fibrotic CAR-T cells reduced fibrosis and restored cardiac function after injury. This approach may hold promise as a therapeutic platform to treat various diseases.
Concluding Remarks
The science of CAR-T therapy and their promise of treating cancer patients is strong. CAR-T therapy is part of the precision medicine revolution which is recognised as the biggest trend of Biotech Market. Currently, there are six FDA approved CAR-T therapies in market and many more are in clinical trials. The Global CAR T cell therapy market opportunity is expected to surpass USD 15 Billion by 2028.
CAR-T therapy is the last resort for patients when all other treatments have failed. The first CAR-T was approved in 2017. Since then we have 6 CAR-T therapies in market for lymphoma, leukemia and multiple myeloma. These CAR-T therapies are directed against either CD19 or BMCA. Now, we have knowledge of more potent target associated antigens, refined CAR development process, more advanced gene editing techniques and more importantly refined techniques to pre-clinically screen CAR-T candidates before going to clinical trial and failing. There is tremendous potential of CAR-T therapy for treatment of blood cancers. In the near future, we can expect a lot more CAR-T therapies approved and curing patients and more importantly keeping them in remission for long term.
CAR T cells have been less effective for solid tumors and brain tumors. Lack of efficacy is most likely multifactorial, but heterogeneous antigen expression; limited migration of T cells to tumor sites; and the immunosuppressive, hostile tumor microenvironment have emerged as major roadblocks that must be addressed. Many companies are attempting to tackle these challenges and generate potent CAR-T cells for solid tumors. Additional genetic modification of CAR T cells (third and fourth generation CARs, TRUCKs, CARs with chimeric switch receptor, split CARs) has greatly enhanced the anti-tumor activity of these cells in preclinical studies, and we hope that some of the devised strategies will translate into improved anti-tumor activity in humans. We have better understanding of tumor associated antigens which will target tumor cells and exclude normal cells. The registered CAR-T cell studies at clinicaltrials.gov target 44 different antigens on solid tumors. To tackle heterogenous tumor antigen expression, BiCistronic CAR-T cells are being tested with promising efficacy in early clinical trials. Besides, at present there are sixteen clinical trials target several antigens at the same time. The top six targeted tumor associated antigens (TAA) that are expressed on solid tumors in many different organs are EGFR, NKG2D-ligands, HER2, B7-H3, MUC1, and CEA. Additionally, we now have discovered more tumor specific antigens with promising data in preclinical trials, ready to be tested in clinical trials.
Other major challenge of CAR-T therapies are they are costly and consume lot of time in production. It is because majority of clinical studies use retroviral or lentiviral vectors to generate clinical-grade CAR T-cell products. To overcome these challenges, non-viral DNA delivery systems are being tested (sleeping beauty transposon system, PiggyBac transposon system, CRISPR-Cas9, or transfection of DNA or RNA), the use of closed-cell manufacturing systems, off-the-shelf allogenic CAR T-cell products also hold the promise to streamline CAR T-cell production and/or distribution.
Several companies are evaluating the efficacy of different immune cells, like γδ (gamma-delta) T cells and invariant natural killer T cells (iNKT), macrophages to target tumor cells. In this regard, Kuur Therapeutics led phase 1 GINAKIT2 study (NCT03294954) of KUR-501 (iNKT cells that express GD2–CARs and IL-15) for patients with R/R high-risk neuroblastoma reported positive interim data of 10 patients treated with single dose of KUS-501. Complete responses and evidence of tumor homing was observed and the CAR-NKT cell therapy was safe and well-tolerated. On May, 2021 Kurt Therapeutics was acquired by Athenex, Inc., (NASDAQ: ATNX. CAR therapy beyond T cells is the new approach to tackle the shortcomings of T cells in regard to different solid tumors.
Combinatorial CAR-T therapy with immunotherapy or monoclonal antibodies helps to circumvent tumor escape and increase anti-tumor activity, they are already clinically tested in many hematologic tumors (and shown encouraging clinical outcome. Such combinations hold great promise for the treatment of solid tumors and are also being tested in clinical trials.
To sum up, let’s look into clinical outcome of 44 clinical trials, total of 375 patients with solid tumors that were treated in these Phase 1/2 trials: complete response was seen in 13, 35 had a partial response, 4 had a mixed response, 121 had a stable disease, 109 had a progressive disease, 8 had no evidence of disease, 5 were not evaluable, and of 80 patients the clinical outcome was not disclosed. In most of the trials, adverse effects were comparable to ones observed with approved CAR-T therapies. However, some events were serious, below figure lists all the adverse effects.

To conclude, CAR-T therapy is in its early teen phase and it has a long way ahead. We remain hopeful that, within the next 5 to 10 years, patients with solid tumors will benefit from CAR T-cell therapies to the same degree that patients with B-cell–derived malignancies do today.
Thanks for reading SciX. Share your views and opinions on the future of CAR-T ?

I'm curious what you think about manufacturing CAR-Ts at scale? There are a number of companies working on automating as much of the process as possible (Lonza, Cellares, Multiply Labs, etc), I'd be interested in your opinion on the issue and what you think the best solutions are.