Dear Readers,
This article will introduce three more Innovator CAR-T therapy companies: ANIX, GRCL, INAB!
7. Anixa Biosciences (Nasdaq: ANIX, M.Cap: USD162.6 M)
Founded in 1982, Anixa is a biotechnology company focused on the treatment and prevention of cancer and infectious diseases. The company was formerly known as ITUS Corporation and changed its name to Anixa Biosciences in October 2018.
Advancements
Use novel type of CAR-T technology, chimeric endocrine receptor T-cell (CER-T) to generate cell therapy portfolio. Their primary focus is to target solid tumors, starting with ovarian cancer, followed by other tumor types.
What is CER-T?
Created by José Conejo-Garcia, a professor and program leader of the Tumor Microenvironment and Metastasis Program at Wistar, now chair of the department of immunology at Moffitt. The CER-T therapy targets the hormone receptor and derives the target-binding domain from its natural ligand. Like, FSH-CER-T will target FSHR-expressing ovarian cancer cells using the full sequence of the FSH hormone. This is unlike CAR-T, which directs T cells by recognising an antibody fragment expressed by tumors.
They have only one autologous CAR-T asset in their pipeline, which targets the antigen Follicle Stimulating Hormone Receptor (FSHR) via CAR carrying hormone called Follicle Stimulating Hormone (FSH) that binds exquisitely to the FSHR.
FSHR is found at immunologically relevant levels exclusively on the granulosa cells of the ovaries and hence it serves as a safe and effective immuno-therapeutic target for ovarian cancer. FSHR is present in 70% of endometrioid carcinomas, 67% of mucinous ovarian carcinomas and 33% of clear cell ovarian carcinomas. The latter two are the most aggressive forms of the cancer.
On Aug 31, 2021 FDA approved the IND application to initiate clinical trial with autologous CAR-T therapy designed with CER-T technology to treat women with relapsed epithelial ovarian cancer. Trial will be led by Robert Wenham, chair of the department of gynecologic oncology at Moffitt Cancer Center.
Expression of FSHR is also observed on the surface of blood vessels in tumors of other types such as prostate, colon, lung, pancreatic and others. Anixa Bio will initiate FSH CAR-T therapy for ovarian cancer patients & if it works they will try this as anti-angiogenic therapy (destroy tumor vasculature and starve or shrink the tumor) in multiple other cancers.
Cancer vaccine portfolio focusses on the immunization against specific “retired” proteins associated with breast cancer (α-lactalbumin) and ovarian cancer (Anti-Mullerian Hormone Receptor 2).
Retired proteins are proteins that are expressed at certain times in life and then are no longer expressed in healthy people.
Besides, they have COVID-9 program, recently company announced that their potential compounds may be even more effective against the Delta variant than the original wild type SARS-CoV-2 as determined by genomic variant analysis.
ANIX’s Vaccine and COVID-19 portfolio will not be discussed in detail here as it is not the focus of this article. However, it is important for investment purpose to understand the company’s orthogonal approach.
Product Pipeline
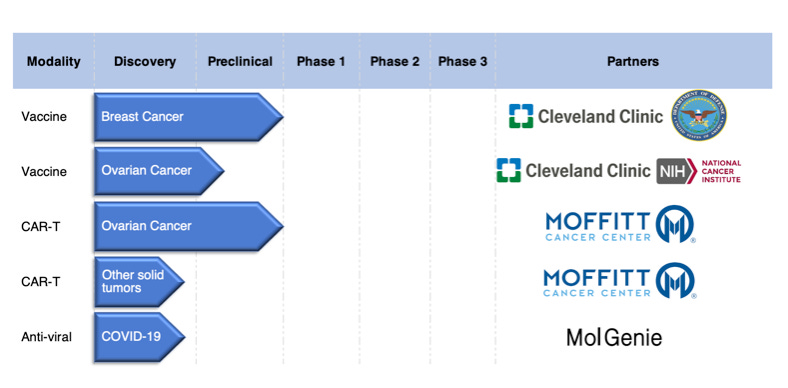
Partners
CER-T technology developed by Moffitt Cancer Center
Breast cancer vaccine trial is in collaboration with Cleveland Clinic and is funded by Department of Defense; Ovarian Cancer pre-clinical R&D is being conducted in NCI
Key management
Chairman, President & Chief Executive Officer: Amit Kumar, PhD
Director: Dr. Arnold Baskies, recently has been appointed to the WHO Global Breast Cancer Initiative.
Financials
The company had US$ 26m in net cash as of the most recent quarter (July-2021) while their quarterly cash burn was US$4m. That implies a cash runway of 1-2 years.
8. Gracell Biotechnologies (Nasdaq: GRCL, M.Cap: USD 756.4 M) - Recent IPO
Founded in 2017 and is headquartered in Suzhou, China. It is a global clinical-stage biopharmaceutical company dedicated to discovering and developing multiple autologous and allogeneic breakthrough cell therapy candidates. Company was listed on NASDAQ on Jan 8, 2021.
Their aim is to overcome major industry challenges, such as lengthy manufacturing time, suboptimal production quality, high therapy cost and lack of effective CAR-T therapies for solid tumors. They have two GMP compliant manufacturing facilities in Suzhou and Shanghai, making them self-sufficient for clinical development and early-stage commercialization.
Advancements
They pioneered FasTCAR and TruUCAR technology platforms.
About FasTCAR:
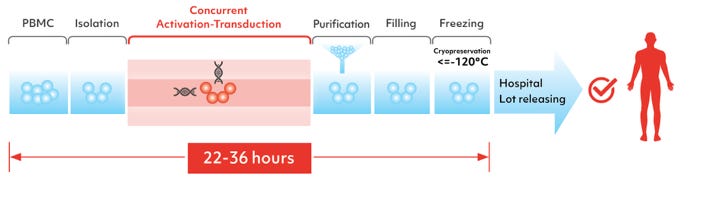
It is an autologous CAR-T program, designed to reduce the manufacturing time and develop less-exhausted CAR-T cells. This is achieved with concurrent activation-transduction step, where rested T cells are simultaneously activated and transduced with XLenti vectors (XLenti are derived from lentivirus, they are of high quality and exhibit high gene transduction efficiency). The ex vivo cell expansion step is not performed, further reducing the autologous CAR-T cell manufacturing time from an industry norm of two to six weeks to 22-36h.
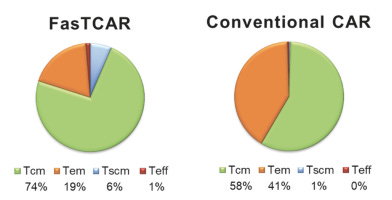
The efficacy of XLenti transduction was demonstrated in pre-clinical studies, it was observed that after transduction, one or more CARs were integrated stably in the T cell genome and were highly active in proliferation and tumor cell clearance. These findings laid the foundation of FasTCAR. In addition, FasTCAR manufactured CAR-T cells are younger, less exhausted and show enhanced proliferation, tissue migration and tumor cell clearance activities.
To produce FasTCAR product candidates, they have designed fully-closed production lines which can reduce the risk of contamination and optimize cost-efficiency. In their closed design system, they are able to operate multiple systems in one manufacturing cleanroom at the same time. This helps to reduce reagent consumable costs, labor costs, workshop equipment operations and depreciation which will further lead to improvement in productivity and scale up of production in a cost-efficient manner.
About TruUCAR:
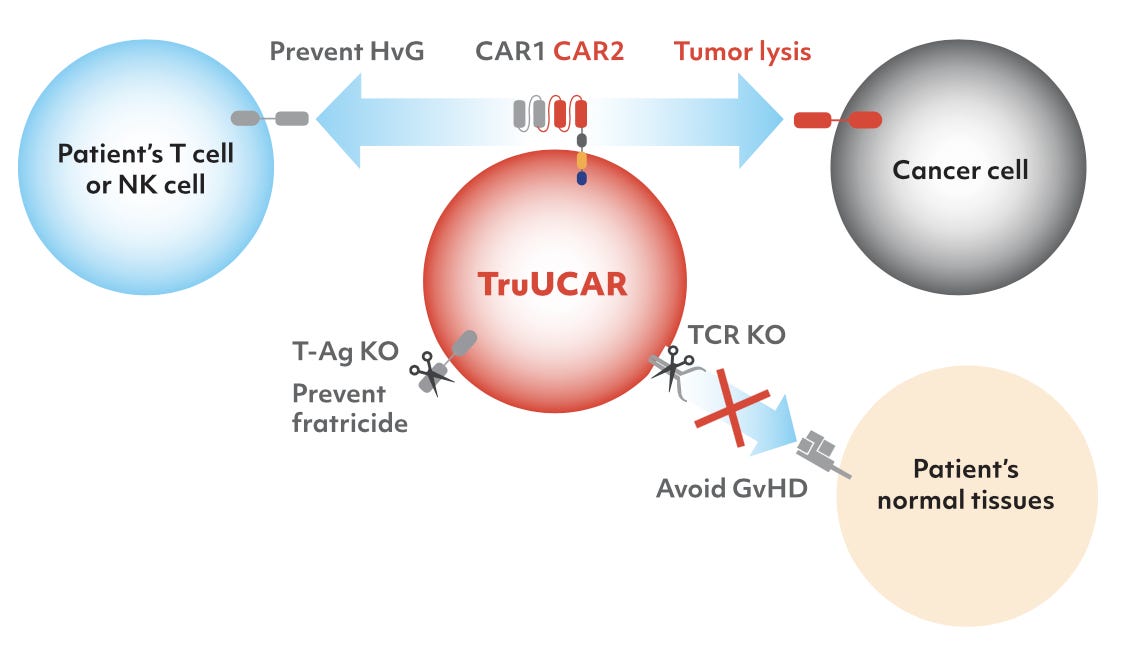
It is allogenic (off-the-shelf) CAR-T platform, TruUCAR is modular, designed to eliminate both HvG and GvHD either by a dual CAR design (one CAR serves a “defensive” purpose and other an “attack” purpose) or a single CAR design for dual functions (the CAR carries out dual functions, targeting both alloreactive killer T cells and NK cells, as well as T cancer cells).
With CRISPR/Cas9, the T cell receptor alpha constant (TRAC) locus is disrupted on TruUCAR product candidates. This helps to reduce GvHD. Furthermore, to eliminate potential fratricide (self-killing of CAR-T cells during the production process), CD7, a pan T and NK marker on the CAR-T cells is also disrupted.
To avoid HvG, TruUCARs are designed to serve a “defensive” purpose, to specifically target patient’s own alloreactive killer T cells and NK cells that would otherwise be directed against the foreign, or allogeneic, cells resulting in rejection by the patients. As a result, TruUCAR’s monotherapy approach has the potential to significantly reduce the cost and length of treatment by achieving fast remission and avoiding anti-CD52 treatment and potentially HSCT.
They have a suite of genetic engineering techniques, Dual CAR and Enhanced CAR, that can be leveraged with FasTCAR and TruUCAR to generate CAR-T cell therapies. Refer back to article, CAR-T in Solid Tumors to recap the benefits of dual CAR and enhanced CAR.
Product Pipeline
Lead assets in FasTCAR and TruUCAR platforms
GC012F: FasTCAR-enabled dual BCMA- and CD19-directed autologous CAR-T product candidate being studied for the treatment of MM in an ongoing investigator-initiated Phase 1 trial across multiple centers in China.
Findings of Ph1 show, 15 of 16 MM patients achieved and maintained a response. In the highest dose cohort which is the recommended dosage level, 100% of the six evaluable patients achieved MRD- sCR as best response which was maintained through the landmark analysis at six months after CAR-T infusion. Based on these results, company expect to submit IND applications for GC012F in r/r MM to the FDA and the NMPA (National Medical Products Administration in China) by the end of 2021.
GC027: TruUCAR-enabled CD7-directed allogeneic CAR-T product candidate being studied for the treatment of adult T-ALL in an ongoing investigator-initiated Phase 1 trial across multiple centers in China.
As of February 2020, five adult r/r T-ALLpatients were enrolled and treated. All five evaluable patients achieved a CR or CRi, resulting in an ORR of 100%, including four patients, or 80%, achieving MRD- CR on Day 28 after treatment. CRS was observed in all patients and was resolved with treatment. No patient developed neurotoxicity or GvHD. It is expected to submit an IND application for GC027 in adult r/r T-ALL to the FDA and the NMPA in 2022.
Partners
Recently signed agreement with Lonza to manufacture Gracell's FasTCAR product candidates in the U.S.
Key management
Founder, Chairman & CEO: Dr. Wei Cao. Dr. Cao has over 30 years of R&D experience in the biotech industry and previously co-founded and served as CEO and executive board member of Cellular Biomedicine Group, Inc. (Nasdaq: CBMG), a Nasdaq-listed cell therapy company.
Financials
As of June 30, 2021, company has US$ 318m in cash and cash equivalents and short-term investments. Net proceeds from IPO: US$ 220m. Their quarterly cash burn is about US$15m so the cash on hand is sufficient for a few years.
9. IN8bio (Nasdaq: INAB, M. Cap: USD 168.8 M) - Recent IPO
Founded in 2016 with the mission to develop novel therapies to address cancer. It is a clinical-stage biopharmaceutical company focused on the discovery, development and commercialisation of gamma-delta T-cell (γδ T cells) product candidates for solid and liquid tumors. IN8bio’s shares of common stock began trading on the Nasdaq Global Market on July 30, 2021. Formerly named as Incysus Therapeutics.
Advancements
DeltEx platform: employs allogeneic, autologous and genetically modified approaches to develop innovative γδ T cell immunotherapies, which are designed to effectively identify and eradicate tumor cells.
About γδ T cells
γδ T cells are defined by the expression of heterodimeric TCRs composed of one γ-chain and one δ-chain, unlike most mature T cells which express the αβ TCR heterodimer.
They represent a relatively small fraction of T cells, <10% of total peripheral blood T cells. However, they are present in substantially greater numbers within epithelial tissues such as skin, intestine, and lung. Resident γδ T cells in tissues defend against pathogens and probably also suppress nascent tumors. But the main γδ T cell in tissues, with a Vδ1 segment for its TCR δ chain, is very hard to grow
Note: GammaDelta Therapeutics, private UK based company focus on γδ T cells residing in tissues to fight cancer; Takeda signed a $100 million deal with GammaDelta in 2017, which includes an option to purchase the company; Adaptate Biotherapeutics, spun-out from GammaDelta Therapeutics in late 2019 is developing an innovative portfolio of therapeutic antibodies designed to modulate the activity of a patient’s own cytotoxic γδ T cells.
γδ T cells are innate effectors: a battle-ready first line of defense against pathogens and cancer, embody properties of both the innate and adaptive immune systems. γδ T cells are capable of recognising and lysing stressed cells (e.g., infected or cancerous cells) in MHC unrestricted manner unlike αβ T-cells. This means that these cells do not require prior antigen priming, have equal potential for killing tumours cells with low mutational loads, are less likely to be affected by therapeutic resistance issues and are suitable for allogeneic therapy as they are not limited by patient-specific factors as are autologous therapies. They are long-lived, can also have immunological memory.
A bioinformatic study of large number of solid tumour samples showed that, amongst all different immune cell types, γδ T cell infiltration correlated highest with survival.

Global prognostic associations for 22 leukocyte types across 25 cancers (n = 5,782 tumors; left) and 14 solid non-brain tumors (n = 3,238 tumors; right); Source: Nature Article Most circulating γδ T cells find their targets by detecting metabolic or other abnormalities like they sense small, nonpeptide antigens called ‘phosphoantigens’, present in infected cells or tumor cells, that ultimately lead to γδ T cell activation. Main type of γδ T cells in circulation are Vγ9Vδ2 cells which are activated by phosphoantigen metabolites of the mevalonate cholesterol biosynthesis pathway. Most of the early clinical trials testing bisphosphonates and bromohydrin pyrophosphate (trial initiated by Innate Pharma) as γδ T activators failed, they proved safe but ineffective (average response ratio of only 21% and an average clinical benefit rate of only 57%).
Now with advanced knowledge about γδ T cells, current trials are designed to overcome the limitations of early clinical trials and test the potential of already proven safe new wave of γδ T cells (for details check out the supplementary section at the end of the article).

Important to know that γδ T cells are double-faced immunocytes that play a role of ‘friend or foe’ in cancer progression; these cells are capable of changing their function in response to cytokine stimulation. Depending on the stimulation by different cytokines in TME, they can change their function toward being either antitumor or protumor. If we properly handle these cells, we benefit from γδ T cell immunotherapy in the long run.

Product Pipeline
INB-100 (DeltEx Allo): an expanded and activated allogeneic γδ T cells product. Simplified development process: Peripheral blood is collected by leukapheresis from the donor, expanded and activated on CliniMACS-Prodigy, further depleted of alpha beta T-cells using the CliniMACS Alpha Beta T-Cell Depletion System, which leaves a gamma delta T-cell rich product.
Phase 1 dose escalation clinical trial of INB-100 conducted at the University of Kansas Cancer Center; NCT03533816. This trial uses γδ T cells to maximize the anti-tumor response and minimize GvHD in leukemic and myelodysplastic patients who have had a partially mismatched bone marrow transplant (haploidentical). Studies conducted by Dr. Lawrence Lamb (co-founder of INAB) have demonstrated a survival benefit in HSCT patients with naturally elevated levels of γδ T cells.
Update on Clinical trial, Aug 11, 2021: The three patients comprising the first cohort did not experience any severe adverse infusion reactions or dose limiting toxicities (DLTs) to date. The first two patients are at 14.5 months and 12.2 months, respectively, post-HSCT as of June 30, 2021, and continue to be in complete remission.
INB-200 (DeltEx Drug Resistant Immunotherapy or DRI): genetically modified autologous to drive improved anti-tumor response for the treatment of solid tumors. INB-200 is engineered to be resistant to alkylating chemotherapy. In GBM patients, to drive improved anti-tumor response INB-200 can be used as an adjuvant with the current standard-of-care, temozolomide. Read the article, to understand mechanistic basics of DRI γδ T cells.
The Phase 1 repeat dose escalation clinical trial of INB-200 is being conducted at the O’Neal Comprehensive Cancer Center at the University of Alabama at Birmingham; NCT04165941. Aim of the trial is to find out if the safety and tolerability of INB-200 is safe to administer to patients with a newly diagnosed GBM in combination with TMZ.

Partners
University of Kansas Cancer Center and University of Alabama at Birmingham
Key management
Lawrence Lamb, PhD: Dr. Lamb was working as the scientific co-founder of Incysus since early 2016 and in 2019 joined as Executive Vice President and Chief Scientific Officer. He is one of the pioneer in γδ T cells immunotherapy research.
Financials
The company is a recent IPO so the financial details are limited. Over the preceding 12m, they had a cash burn of US$9m. In their just concluded IPO, they raised $40m and the stock started trading on 30-July-2021. The company had previously attempted to IPO in late 2020 but that did not then materialise.
Supplementary Info
There’s a new burst of interest in γδ T cells, and several companies are in the race to unleash the power of unconventional γδ T cells against cancer. Recently, Imcheck Therapeutics (private company, based in France) announced $53 million financing round co-led by Pfizer Ventures which has further heightened the buzz around γδ T cells.
Below is the list of ongoing trials in this space: