Dear Readers,
Let us explore the CAR-T innovators’ landscape further (viz. SRNE, ZIOP, MBIO)
Innovator Companies Continued…
4. Sorrento Therapeutics (Nasdaq: SRNE, M.cap: USD 2.7b)
Founded in 2009, this company is focussed to create novel therapies to improve lives of people with cancer and intractable pain. Sorrento is also developing potential antiviral therapies and vaccines against COVID-19. They have a diverse oncology portfolio which includes immune checkpoint inhibitors, bispecific antibodies (G-MAB™ library), antibody-drug conjugates, CARs and dimeric antigen receptor (DAR) based cellular therapies, and oncolytic viruses. Company’s fully human antibodies include PD-1, PD-L1, CD38, CD123, CD47, BCMA, LAG3, CTLA-4, CD137 and SARS-CoV-2 neutralising antibodies, among others. Their strategy is to test the potential of each asset individually and also as a combination. Combinatorial therapy approach may have the potential to break-through the most difficult cancer challenges, especially in solid tumors. They have 6 cGMP Manufacturing Sites, which include state-of-the-art cGMP antibody and cell therapy manufacturing facility (located in San Diego, CA), Oncolytic Virus Production and ADC Conjugation (Camino Santa Fe), Payload and Linker Synthesis (Suzhou, China), etc.
Advancements
Their CAR-T program includes CARs against CD38, Carcinoembryonic antigen (CEA) and CD123 antigens.
Phase 1b study of the efficacy and safety of CAR2 Anti-CEA CAR-T Cells for Pancreatic carcinoma patients with CEA, liver metastases and resistant to standard therapy is under progress; NCT03818165
CEA is expressed in up to 70% of pancreatic cancers, and ability to measure its levels in serum leading to its potential as a tumor specific antigenic target. Its expression profile has made it a target of choice for vaccine trials. Preclinical studies done in murine models of PDAC have also shown promising long-term anti-tumor responses with CEA-redirected CAR T cells. However, it should be noted that CEA is also expressed in low levels on some healthy tissues and off-target effects can be expected, toxicities in humans have been reported with first generation CAR-T directed against CD38.
Besides CEA, other candidate target antigens in clinical trials for CAR T cell therapy in pancreatic ductal adenocarcinoma are CD24, prostate stem cell antigen (PSCA), Muc-1, Mesothelin, FAP, prominin 1 (PROM1) and human epidermal growth factor receptor 2 (HER2). Some very new target antigens are CD66c, CD318 and TSPAN8, still being tested in vivo pre-clinical trials.
Phase 1 single dose-escalation safety study of CAR2 Anti-CD38 A2 CAR-T Cells in patients with Relapsed or Refractory Multiple Myeloma (MM) is also under progress; NCT03464916
CD38 is considered as suitable target for MM due to its high and uniform expression on all malignant plasma cells regardless of the spectrum of disease (ex. MGUS, SMM, active MM). Its choice as a potential target is supported by the clinical success of CD38-specific human monoclonal antibody daratumumab and the second-generation isatuximab together with promising anti-myeloma effects observed in preclinical studies.
It is noteworthy that CD38 expression is downregulated in advanced disease and thus resistance to the anti-CD38-CAR-T may be expected. CD38 is also expressed on activated T cells, NK cells and normal prostate, neuronal, and muscle cells so there is also a likelihood for on-target/off-tumor toxicity. Other more promising targets for R/R MM under clinical trials are BCMA, SLAMF7/CS1, TACI, CD138. If interested to learn more about CAR-T therapy in MM, click here.
The company is assessing CD123 CAR T in acute myeloid leukemia (AML). Cellectis’ is also evaluating the safety and dosing of CD123 CAR-T in Ph 1 for AML.
Besides, CAR assets, they have DAR-T cells.
What are DAR-T ? The DAR or Dimeric Antigen Receptor utilises a natural antibody Fab (antigen-binding fragment) instead of the scFv (single-chain variable fragment) used by traditional Chimeric Antigen Receptor (CAR) T cells. DAR-Ts are genetically engineered normal healthy donor derived T cells (allogenic), proprietary non-viral knock-out knock-in (KOKI) technology is utilised to express the DAR into T-cell receptor (TCR) alpha chain constant region (TRAC). In this manner, TRAC is knocked out and antigen is knocked into its locus.
KOKI technology generated DARs enables an off-the-shelf treatment approach, thereby eliminating the need for patients to undergo leukapheresis and undesirable treatment delays to perform cell harvesting, manufacturing and release prior to treatment for each individual cancer patient.
The non-viral KOKI technology may also offer other potential advantages over existing virus-based technology such as transgene-encoding lentiviruses or retrovirus streamlined method for transgene construct production without need for laborious and time-consuming virus production, release and validation processes which can result in a shorter research and development timelines for IND-enabling activities.
Preclinical studies demonstrate improved target specificity, stability and functionality of DARs over traditional CARs, due to higher inherent stability of the Fab and stronger affinity. Greater specificity also reduces potential undesirable side effects, i.e. eliminating the possibility of GvHD and toxicity (CRS).
CD38 DAR-T (Allogenic) is the first allogeneic candidate based on Sorrento’s proprietary DAR–T cell platform. Sorrento received FDA authorization in Aug 2021 to start Phase 1 clinical trial for CD38-DAR in MM patients.
Product Pipeline
Partners
Department of Medicine, Huddinge, Karolinska Institutet (KI) agreement to develop the iPSC-derived NK cell-based cancer immunotherapies (“NextGenNK”). The foundational Sorrento research assets critical to this program are novel proprietary CAR and DAR constructs identified through Sorrento’s proprietary G-MAB™ fully human antibody library and previously validated as determinants of cell-based therapy potency against hematologic and solid tumors. KI will bring in their expertise in the field of NK cell therapy.
Sorrento acquired ACEA Therapeutics on June 1, 2021. ACEA is developing multiple clinical and preclinical-stage new chemical entity compounds, including the late clinical drug candidate, Abivertinib. It has operations in both China and the United States.
Financials
As of June 30, 2021, they have a Net debt of US$ 35m while net loss was US$ 166.7m in that quarter. They clearly need more capital.
5. Ziopharm Oncology (Nasdaq: ZIOP, M.Cap USD 381m)
Founded in 1998 by Jonathan Lewis; Ziopharm is driven to reinvent bioengineering and manufacturing processes to develop next generation of immunotherapies to treat both haematological and solid cancers. They employ non-viral and cytokine-driven cell approaches to generate low cost and potent CAR-T therapy and also aim to combat the complexity of delivering gene and cell therapies. The Company has been implementing a strategy to build in-house cGMP clinical production capabilities at the Company’s facility in Houston, TX.
Advancements
Utilises the best-in-class non-viral Sleeping Beauty (SB)-based transposon (which does not require T cell replication) for rapid gene insertion. As compared to viral gene transfer, this gene transfer method is less costly, easy and simple.
Ziopharm’s bioengineering approach has following key components:
Improve T cell persistence, survival advantage and stave off immune cell exhaustion: Interleukin 15 is tethered to the T-cells’ membranes, and this membrane-bound IL-15 or mbIL-15 is designed to give support to T cells in TME. Preclinical data have shown persistence of the least differentiated memory T cell, the T-memory stem cell, which was promoted by signaling induced by a membrane-bound chimeric IL-15 cytokine-fusion molecule.
Auto-Control: molecular safety switch is inserted into the DNA, giving control to deactivate the modified T cells as needed.
With Rapid Personalised Manufacturing approach (third generation SB Trial with improved SB system), company will have the ability to very rapidly manufacture onsite genetically modified CAR T cells in less than two days.
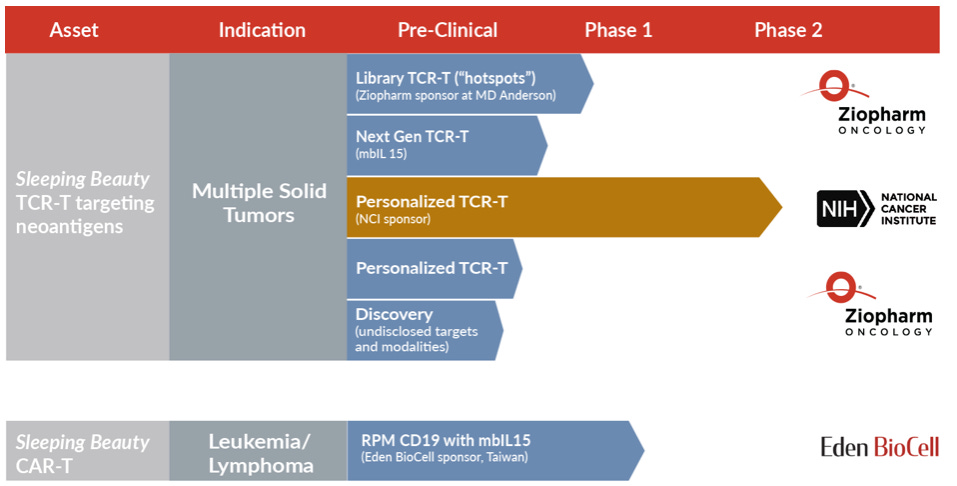
Specificity to reduce the off-target effects: using SB to genetically modify TCRs to target neoantigens (products of mutated genes) to make a truly personalized cell therapy. Neoantigens are typically unique to each patient’s cancer and not present on any healthy cells or tissue. They are the blueprint and “Achilles heel” for effective targeting of all cancers. To learn more about Sleeping Beauty transposition of mutation-specific TCRs targeting unique neoantigens, click here.
TCR-T program: multiple TCRs with unique specificities targeting recurrent p53 and KRAS substitutions in frequent HLA haplotypes are stably expressed using Sleeping Beauty transposition in healthy peripheral blood T-cells to re-direct towards tumor cells. The primary difference between the CAR T and TCR T approach lies in whether the engineered T cell product recognizes cancer proteins expressed on the surface of the cell (CAR T) or inside the cells (TCR T).
Important to note that solid tumors are heterogenous and thus it will be crucial to select the target neoantigens …as depending on how many mutations the therapy targets, the efficacy potential can be compromised.
Pre-clinical and clinical trials: preclinical data presented in AACR meeting. Phase I/2 clinical trial has been cleared by the FDA, and will evaluate the ability of a library of autologous KRAS and p53 neoantigen-specific TCR-T cells to eliminate R/R tumors in adult patients with a number of cancer types including lung cancer, gynecological cancers, colorectal cancer, pancreatic cancer and cholangiocarcinoma.
A preliminary clinical study following hematopoietic stem cell transplant has provided encouraging support for SB-based gene insertion.
Another Phase1 clinical trial study conducted by Eden BioCell with CD19 RPM CAR-T have dosed 2 patients since trial’s initiation in March 2021. No serious side effects were observed. This trial was financially supported by TriArm Therapeutics in Taiwan.
Clinical data demonstrated safety, tolerability, disease response, long-term multi-year survival of patients with NHL (OS 100%) and ALL (OS 49%), and persistence (currently, detected for up to 4 years in some recipients) of infused CD19-specific CAR+ T cells.
Approach to turn Cold (devoid of tumor attacking immune cells:immunological desert) to Hot Tumors—Controlled IL-12: developing IL-12 coded within replication incompetent adenovirus (Ad-RTS-hIL-12 plus veledimex; under the control of the RheoSwitch Therapeutic System) to be delivered at tumor site which will then signal killer T cells to migrate to tumor site (hot tumor) and begin a sustained attack. This strategy is now employed to treat patients with Glioblastoma (Brain tumors) in combination with checkpoint inhibitors and its therapeutic potential will also be explored in other solid tumors.
Product Pipeline
Partners
National Cancer Institute, The University of Texas, MD Anderson Cancer Center and Eden BioCell: for development of Sleeping Beauty platform; novel TCR therapies, CAR-T therapies, non-viral gene transfer systems, genetic modification and/or propagation approaches arose from the laboratory of Laurence Cooper, M.D., Ph.D., MD Anderson. In 2015, Dr. Cooper became Ziopharm’s Chief Executive Officer after Ziopharm licensed this foundational technology from MD Anderson.
The NCI under the leadership of Steven Rosenberg, M.D. will conduct a Phase 1 trial to evaluate adoptive cell transfer immunotherapies using the Sleeping Beauty transposon/transposase system to express TCRs for the treatment of solid tumors. These genetically modified TCRs are designed to recognize and attack specific neoantigens expressed within a patient’s cancer.
Regeneron Pharmaceuticals: agreement for controlled IL-12 platform.
Key management
Carl June, M.D., pioneer of CAR-T therapy is part of scientific advisory board and Dr. Steven Rosenberg, Key Research Partner on TCR Work. On July 2019, Dr. Drew Deniger of NCI joined Ziopharm to Direct TCR-T Cell Therapy Program. Dr Deniger is recognised leader in the identification of neoantigens in hotspots, advancing innovative immunotherapy approaches into the clinic, and with years of expertise with the Sleeping Beauty system.
Company appointed Kevin S. Boyle,Sr. as its new CEO on Aug 30, 2021. Mr. Boyle was CEO of Kuur Therapeutics (formerly known as Cell Medica Ltd.).
Financials
As of June 30, 2021, the Company had approximately US$71m of net cash on its balance sheet. It’s quarterly cash burn is around US$22m which gives them a cash runway of 6-12 months.
6. Mustang Bio (NASDAQ: MBIO, M.Cap: USD 278m)
Company was incorporated in 2015, it is a clinical-stage biopharmaceutical company focussed on developing next-generation therapies for hematologic cancers, glioblastoma and rare genetic diseases. Mustang was founded by Fortress Biotech, Inc. (NASDAQ: FBIO). Company’s strategy is to license the CAR T therapies being developed under exclusive licenses from several world class research institutions. They support preclinical and clinical research activities of partners and then transfer the underlying manufacturing technology to their cell processing facility to conduct our own clinical trials.
Advancements
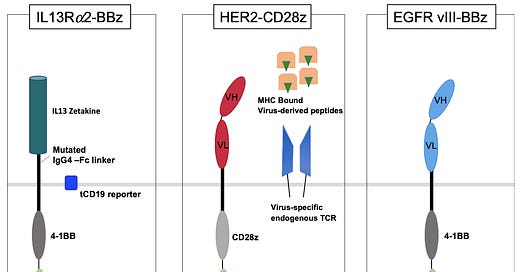
Autologous or Allogeneic CD123+ CAR T cells (MB-102): Genetically modified using a self-inactivating (SIN) lentiviral vector to express CD123CAR (which contains an anti-CD123 scFv, an optimized IgG4 CH2CH3 linker, a CD28 co-stimulatory domain, and a CD3 zeta signaling domain) as well as a truncated EGFR (CD123CAR-CD28-CD3zeta-EGFRt+ T cells).
CD123 is an attractive target for T cell based adoptive immunotherapy as it is over expressed on AML blasts and leukemic stem cell (LSC)-enriched cell sub-populations compared to normal hematopoietic stem cells and myeloid progenitors and over 90% in Blastic plasmacytoid dendritic cell neoplasm (BPDCN) patients
City of Hope (CoH) sponsored Phase 1/2 clinical trial of MB-102 for the treatment of CD123+ r/r AML and BPCDN is ongoing (NCT02159495). CoH presented the early data of this trial on ASH conference, 2017. They observed promising anti-leukemic activity in both AML and BPDCN with no myeloablative effects. Recently they also started a phase 1/2 study to assess the safety and efficacy of MB-102 in patients with relapsed or refractory BPDCN (NCT04109482). MB-102 has received Orphan Drug Designations from the U.S. Food and Drug Administration for AML and BPDCN.
Lentiviral vector encoding CD20 targeting CAR-T with both 4-1BB and CD28 co-stimulatory domains (MB-106). Fred Hutchinson Cancer Research Center sponsored trial of MB-106 in CD20+ non-Hodgkin lymphoma and chronic lymphocytic leukemia patients is ongoing (NCT03277729). Early findings have shown promising findings, it will be interesting to follow up the clinical trial.
CAR-T pipeline for Solid Tumors: targeting IL13Ra2 (MB-101), HER2 (MB-103) and prostate stem cell antigen (MB-105) are being developed in partnership with City of Hope.
IL13Rα2 is an attractive and promising target for GBM as it is specifically overexpressed in GBM but not normal tissue and with higher affinity binding sites than IL13Rα1.
IL13Rα2L CAR-T design lentivirally transduced CD62L-enriched central memory T cells (Tcm) to express an IL13Rα2-Specific, Hinge-Optimized, 41BB-Co-stimulatory chimeric receptor and a truncated CD19. Phase 1 trial for patients with r/r GBM is ongoing (NCT02208362). Initial findings demonstrated positive response.

CAR constructs used in early studies for treatment of GBM; Source: Review Despite the initial positive response in clinical trials, IL13Rα2-specific CAR T-cell therapy is being challenged by tumor recurrence due to antigen loss and limited T cell persistence. Three approaches CoH is adopting to increase the treatment efficacy of MB-101 for GBM patients:
Careful selection of patients: Trial ongoing to test of CAR-T are effective for leptomeningeal disease (NCT04661384);
Make tumors hot (immune-rich) by combining with C134 oncolytic virus, MB-108 ( in partnership with Nationwide. C134 is genetically modified oncolytic Herpes Simplex Virus (oHSV) to kill tumor cells and spare normal healthy cells, this in turn induces an immune response to fight the cancer.
MB-101 has shown a promising early response rate in CoH sponsored trial and trial of C134 in patients with recurrent GBM is also ongoing (NCT03657576). Company also plans to submit IND application for combinatorial therapy (MB-108 and MB-101) in 4Q2021.
Combine with checkpoint inhibitors to overcome PD1-mediated immunosuppression. Combination trial with nivolumab and Ipilimumab is ongoing (NCT04003649).
HER2 CAR-T (MB-103): Lentiviral transduced memory enriched T cells to express HER2(EQ)BBζ/CD19t+ T cells. CoH led trial to evaluate the side effects and best dose of MB-103 is ongoing (NCT03389230 and NCT03696030). HER2 is overexpressed in many cancer types including breast, ovarian and GBM, however it also is expressed in some normal tissues, leading to safety concerns. In this case design of CAR becomes crucial, check the article to understand more.
Product Pipeline
Partners
Active collaborations with City of Hope (Dr. Christine Brown and Dr. Stephen Forman); Fred Hutchinson Cancer Research Center (Dr. Brian Till); Nationwide (Dr. Kevin Cassady); and St. Jude Children’s Research Hospital (Dr. Brian Sorrentino).
Key management
Leadership team with extensive background in gene, cell and rare disease therapy.
Financials
As of June 30, 2021, the Company has a net cash position of US$ 128m while it’s quarterly cash burn has been around $14m in that quarter. That suggests a cash runway of 1-2 years. With 40% market cap as cash, it seems interesting.