Dear Readers,
Welcome to SciX Newsletter. If you are new to SciX and want to get a view of what to expect in the articles, please click here.
Here, we will explore another recent member of the CAR cell therapy world-Regulatory T cells (CAR-Treg). Tregs are specialized branch of CD4+ T lymphocytes endowed with immunomodulatory functions which include maintaining immune homeostasis and immune tolerance, as well as regulating proinflammatory immune responses. Therefore, Treg cells can be utilised as an ideal cellular therapeutic tool for treatment of autoimmune diseases, as well as allogeneic hematopoietic cell or solid organ transplant thus reducing dependency on immunosuppressive drugs. Now, with advanced genome editing techniques, it is possible to generate disease-relevant antigen-specific regulatory T cells in large numbers for autoimmune, GvHD, inflammatory diseases like Inflammatory Bowel Disease, Rheumatoid Arthritis, Crohn’s Disease. The scientific foundations of CAR-Treg development are built on the lessons learned from the success of CAR-T therapy for haematological cancers.
This article will cover the Treg market space, here we will:
Explore the reasons of introducing a CAR to Tregs
Applications of Treg based therapy
Get familiar with the leading and emerging biotech companies/startups (Sangamo Therapeutics, Quell Therapeutics, GentiBio, Abata Therapeutics, Sonoma Biotherapeutics, AZTherapies and TeraImmune) in the CAR or TCR-Treg space
Learn about the lead assets of different companies and outline the scientific approach behind their development.
Reflect on the investment theory, clinical trials, market opportunities, and risks involved
Touch upon the financials of each company.
Challenges
Conclusions

If you haven’t subscribed yet, please do so now. Subscribers receive every post of SciX newsletter directly in their inbox and an opportunity to ask questions and submit suggestions!
1.1 Regulatory T cells (TReg)
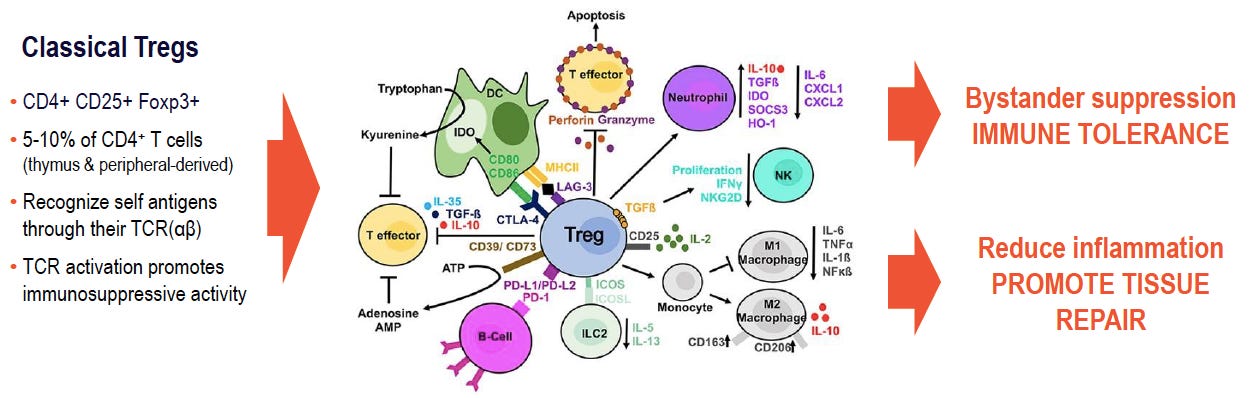
Tregs are a subset of T cells that are essential for maintaining peripheral tolerance, preventing autoimmune diseases and limiting chronic inflammatory diseases. They comprise 5%-10% of the CD4+ T cell population. There are two major subsets of Treg cells: natural Treg (nTreg) cells that develop in the thymus, and induced Treg (iTreg) cells that arise in the periphery from CD4+Foxp3- conventional T cells, which can be generated in vitro. Although they have slightly different phenotypes, their suppressive function is known to be very similar.
Tregs mediate their suppressive effects by different mechanisms which can be classified into the following four basic 'modes of action':
Suppression by inhibitory cytokines-like Interleukin-10 (IL-10), Transforming Growth Factor-beta (TGF-b) and IL-35,
Suppression by cytolysis via granzyme A and/or granzyme B and perforin,
Suppression by metabolic disruption and
Suppression by modulation of dendritic-cell maturation or function via either through cytotoxic T-lymphocyte antigen 4 (CTLA4)–CD80/CD86 interactions and lymphocyte-activation gene 3 (LAG3) binding to MHC class II molecules.
1.2 Why Tregs need a CAR:
Tregs are powerful suppressive cells, they can broadly suppress T cells with differing antigen specificity. However, this non-specific immune-suppression may result in nonspecific tolerance which is known to limit robust immune responses when necessary. Additionally, a major hurdle for nonengineered Tregs is their conversion into proinflammatory T helper 17 cells (Th17) cells in response to certain immunological environments.
One strategy to engineer specificity in polyclonal Tregs is to express a CAR, which is a synthetic molecule that combines extracellular single-chain variable fragments (scFvs) of an antibody with primary T cell receptor (TCR) signaling and costimulatory moieties. Alternatively, this strategy can potentially be applied to FoxP3-engineered conventional T cells under conditions of Treg scarcity.
CAR engineered (antigen-specific) Tregs are reported to be more potent than polyclonal conventional Tregs. Furthermore, when compared to TCR-Tregs (Tregs engineered with specific TCR), CAR-Tregs recognize only the extracellular antigen and are also MHC-independent. Furthermore, CAR Tregs are less dependent on IL-2 than TCR-Tregs.
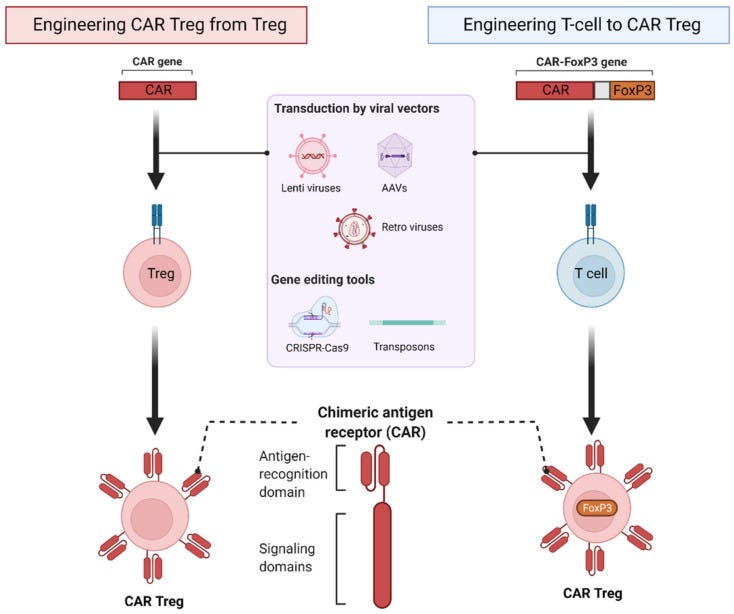
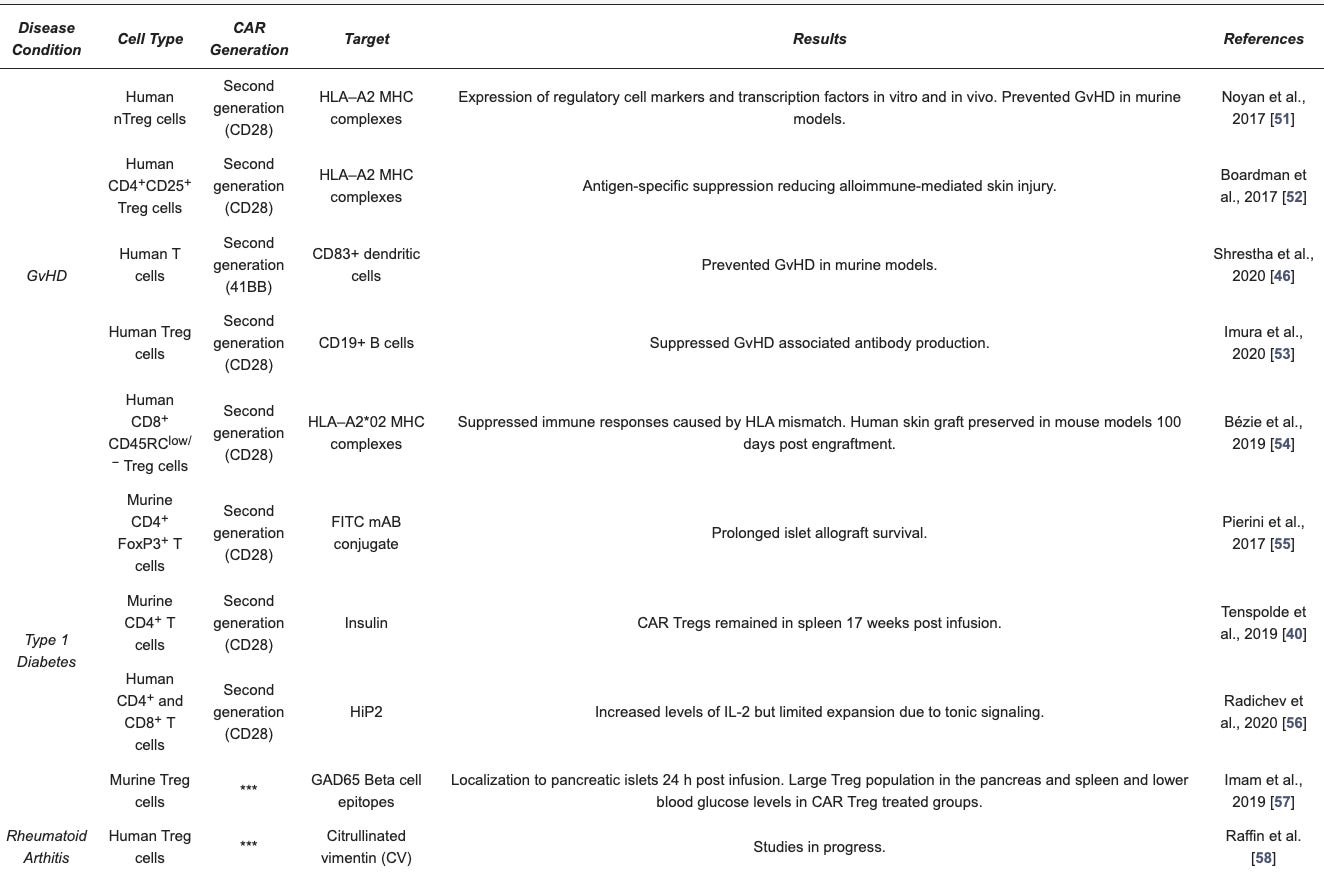
Collectively, several preclinical studies demonstrated the feasibility and functionality of engineered Treg cells (see Table 1). First-in-human trials with first-generation natural Treg cell products have yielded promising data regarding safety and efficacy in immune-related disorders. The possibility to successfully modulate the immune system to facilitate tolerance development in autoimmune diseases, allows allogeneic organ transplant and minimise use of immunosuppressive drugs is one of the top achievement of modern immunology.
2. Applications for CAR-Treg therapy
So far, pre-clinical studies with CAR Tregs show promise in models of autoimmune disease and allograft rejection with a first-in-man clinical trial for kidney transplantation. CAR-Tregs can find application in different medical conditions. Below is the list of medical conditions with the name of the companies focused on developing CAR-Treg therapies:
Graft versus Host Disease (GvHD):
Sangamo Therapeutics developing CAR Treg for kidney transplant patients (STeadfast, first CAR Treg PhI/II Clinical Trial, NCT04817774) and
Quell Therapeutics focused on CAR Tregs for liver transplant recipients (LIBERATE, PhI/II Clinical Trial, NCT05234190).
Type I Diabetes (T1D): GentiBio and Abata Therapeutics
Rheumatoid Athritis: Sonoma Biotherapeutics
Multiple Sclerosis: Abata Therapeutics and TeraImmune
Inflammatory Bowel Disease: Sangamo Therapeutics developing Tregs with a CAR targeted to IL23R for Crohn’s disease patients.
Asthma
Allergy
Atopic dermatitis, Eczema: TeraImmune
Haemophilia: TeraImmune
Tregs, in their natural form, have been evaluated in dozens of clinical trials to date. Isolated from the blood, grown in cell culture and then reintroduced into patients, these cell therapies seem to be generally safe, with hints of efficacy in some transplant settings.

3. Companies developing CAR-Tregs
In recent years, Treg based therapies have become a research hotspot in the field of immunotherapy. Treg cells has gained a wealth of interest in recent years as companies look to tamp down overactive immune systems. Big Pharma Companies are either acquiring or investing in startups developing Treg based therapies. In February 2021, Merck NYSE: MRK) signed away $1.85 B to acquire Pandion Pharmaceuticals (Nasdaq: PAND) and its pipeline of drugs targeting Tregs for autoimmune conditions. Other Big Pharma giants like Eli Lilly (LLY), J&J (JNJ) and Pfizer (PFE) invested in South San Francisco-based TRexBio, a startup mapping Tregs in human tissue to identify targets in oncology and autoimmune disorders. Another Pharma Giant, Novartis (NVS) is financially supporting the Treg pipeline of GentiBio, a Seattle based startup.
More than 90 companies and academic / research institutes are developing Treg based product candidates like Treg enhancing biologics and drug programs (histone deacetylase inhibitors, p38 MAPK inhibitors, and IL-2-based therapies), autologous Treg therapies, allogeneic Treg derived exosomes, TCR-Tregs, and CAR-Tregs against multiple target indications. Non-engineered Treg assets of Coya Therapeutics (Nasdaq: COYA), Caladrius Biosciences (Nasdaq: CLBS), ILTOO Pharma, Eli Lilly (LLY), REGiMMUNE (TPEx) are in advanced stages of development.
It is worth highlighting that, in the last five years, capital investments worth around US$ 3B have been made by strategic investors in the Treg therapy domain.
Here, we will focus on companies/startups developing engineered CAR-Treg or TCR-Treg therapies.
3.1 Sangamo Therapeutics (NASDAQ: SGMO, M. Cap= $595 M)
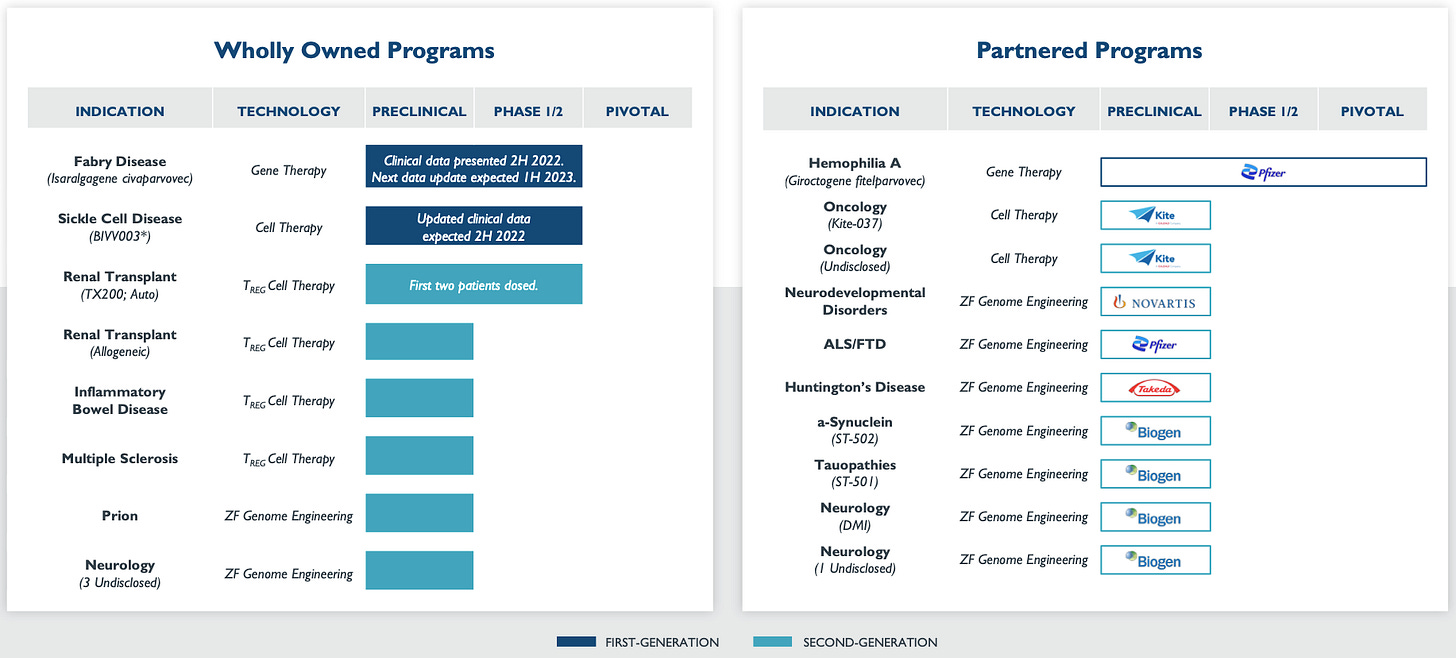
Sangamo Tx is a Genomic Medicines Company, they acquired the CAR-Treg specialist Txcell in 2018 and became the first company to treat a patient with engineered CAR-Tregs. They have robust pipeline of cell therapy assets (see below chart).
Lead Asset:
TX200-TR101 is an autologous gene therapy composed of Treg cells (CD4+/CD45RA+/CD25+/CD127low/neg) that have been ex vivo expanded and transduced with a self-inactivating lentiviral vector encoding for a CAR to recognize HLA-A2 coupled to a transmembrane domain and the activation domain of human CD28 and human CD3 zeta (third-generation CAR).
First-in-human CAR-Treg STEADFAST clinical trial (NCT0481777): The purpose of this study is to evaluate the safety and tolerability of TX200-TR101 and its effects on the donated kidney in living donor kidney transplant recipients. Study aims for the CAR-Tregs to recognise the HLA-A2 molecule present on the donated kidney and subsequently induce and maintain immunological tolerance to the organ.
Till now two patients have been dosed in the Phase 1/2 STEADFAST. TX200 continues to be generally well tolerated in both patients.
Unmet medical need:
Renal transplantation patients require lifelong immunosuppressive therapy, which is frequently associated with adverse side-effects, such as high risk of severe infections, cardiovascular disease, and malignancy. Therefore, an important goal in transplantation medicine is the creation of immune tolerance aiming at allograft acceptance while enabling reduction of immunosuppressive therapy.
Mismatched HLAs between donor and graft recipients are a major barrier to successful transplantation. Approximately 70% of white transplant recipients are HLA-A02 negative and 30% of organ donors are HLA-A02 positive, leading to a potential mismatch in 21% to 26% of transplantations. Company aims to promote immunological tolerance to renal graft, maintain allograft acceptance with autologous CAR-Tregs therapy while also reducing the dependence on immunosuppressive therapy.
Other Assets in pipeline:
IL-23R-CAR-Treg for Crohn’s disease (CD): CD is an IBD which is characterized by a chronic relapsing and remitting inflammation of the gastrointestinal tract. IL23 is a key player in the pathogenesis of inflammatory bowel disease (IBD). When the CAR-Tregs are infused into the patient, they are expected to migrate to the GI tract, engage IL23R and suppress the local inflammation.
Company is also working on developing Allogeneic CAR-Tregs, which will be engineered using a donor DNA consisting of a CAR-expressing adeno-associated virus type 6 (CAR-AAV6) and zinc finger nuclease (ZFN) editing technology to insert the CAR into the beta-2 microglobulin (B2M) locus, leading to the knockout of cell-surface expression of the MHC-I.
Market Opportunity:
Approximately 46,000 renal transplantations expected in US and EU (2021 data). About 21-26% of transplanted organs are estimated to be HLA-A2 mismatched.
Key Risks:
Highly innovative concept in an emerging space and complex manufacturing
Financials: (Taken from Third Quarter 2022 Financial Results)
Revenues for the third quarter ended September 30, 2022 were $26.5 million, compared to $28.6 million for the same period in 2021.
3.2 Quell Therapeutics (Private, raised a total of $240M)
Based in London, UK the company was founded in 2019 in partnership with six prominent immunology experts based in 3 prestigious academic institutes (KCL, UCL, Hannover). Company is led by Iain McGill who brings 25 years of experience in the pharmaceutical and biotech sector.
Investment Thesis
Company is developing engineered Treg therapies to address a range of autoimmune and inflammatory diseases, as well as preventing rejection in organ transplantation.
Lead Asset:
QEL-001 (HLA-A2 CAR-Treg): QEL-001 is an autologous therapy that is composed of engineered regulatory T cells transduced with a lentiviral vector containing a CAR directed against HLA-A2. QEL-001’s Tregs are engineered with CAR containing two unique properties-‘phenotype lock’ which keep the Tregs looking and functioning like Tregs and ‘safety switch’ which company licensed “from the world of oncology”.
PhI/II Clinical Trial (NCT05234190): study of an autologous CAR-Treg in HLA-A2 mismatched liver transplant recipients. The aim is for the CAR-Tregs to be activated on recognition of HLA-A2 antigens present on the donated liver and subsequently induce and maintain immunological tolerance to the organ.
To expand company’s pipeline and develop off-the-shelf Treg products, on April 2022, Quell Therapeutics and Cellistic enter a strategic collaboration to develop an iPSC-derived allogeneic Treg cell therapy platform.
Unmet medical need:
Current standard of care for prevention of liver transplant rejection is to receive a cocktail of immunosuppressive medications such as corticosteroids, calcineurin inhibitors (such as tacrolimus), plus an antiproliferative agent (such as mycophenolate mofetil). However, this cocktail of medications is extremely potent and suppresses the patient’s immune system globally, resulting in an array of serious long-term side effects, such as toxicity, infections and sometimes cancer in long term, significantly impacting patient quality of life.
Market Opportunity:
15,000 liver transplants p.a across US and Europe
Key Risks:
Highly innovative concept in an emerging space and Complex manufacturing
Financials:
Quell Therapeutics, seeded by healthcare company creator Syncona, has now raised a total of $240.5M in funding over 3 rounds. Their latest funding was raised on Nov 29, 2021 from a Series B round. Ridgeback Capital and Jeito Capital are the most recent investors.
3.3 Sonoma Biotherapeutics (Private, raised a total of $335M)
Sonoma Biotherapeutics, an immune tolerance company is based in South San Francisco and Seattle. It was founded in 2020 by pioneers in Treg biology and cell therapy. Cofounders Drs. Alexander Rudensky and Sonoma Bio’s CSO, Fred Ramsdell, co-discovered FOXP3, a critical transcription factor of Treg cells development and function. Drs. Jeffery Bluestone, CEO and Qizhi Tang co-founders of Sonoma Bio have pioneered adoptive Treg cell therapy in autoimmune diseases and organ transplantation.
Investment Thesis
The company is employing proprietary platform technologies (Treg cell therapy platform and a novel Teff conditioning biologic program) and approaches to develop a new generation of adoptive Treg cell therapies alone and in combination with Teff conditioning therapy to induce durable immune tolerance to treat and prevent autoimmune and inflammatory diseases.
Lead Asset
SBT-11-5301 is a CD2-binding fusion protein that resets the microenvironment by selectively depleting and inactivating these Teff cells at the site of disease. As a combination therapy, SBT115301 is designed to pre-condition patients by clearing away overactive, disease-driving effector T cells for optimal treatment with Treg cell therapies.
On June, 2022 FDA cleared Investigational New Drug (IND) Application for SBT115301, an Effector T Cell-Modulating Biologic for Autoimmune Diseases.
SBT-77-7101 is a novel CAR-Treg therapy for patients with refractory rheumatoid arthritis.
Sonoma CSO Fred Ramsdell contends that Tregs are the best cell type for therapeutics. They maintain native TCR signaling pathways and the usual epigenetic identity, he points out, which should ensure proper long-term function and consistency.
Market Opportunity:
According to NIH, more than 24M people in the US and approximately 4% of the world’s population is impacted by autoimmune diseases (Type I Diabetes, Multiple Sclerosis, Rheumatoid arthritis, etc). The treatments available to these patients often have short-lived efficacy, need to be chronically administered and most importantly does not treat the underlying cause of the disease.
Financials:
Sonoma BioTherapeutics has raised a total of $335M in funding over 3 rounds. Their latest funding was raised on Aug 4, 2021 from a Series B round. Sonoma BioTherapeutics is funded by 23 investors. Piper Sandler and Frazier Healthcare Partners are the most recent investors.
3.4 GentiBio (Private, raised a total of $177M)
Based in Seattle, US, GentiBio is a biotherapeutics company co-founded by pioneers in Treg biology and immunology from Seattle Children’s Research Institute, Benaroya Research Institute, and MIGAL Galilee Research Institute. Company is developing engineered regulatory T cells programmed to treat autoimmune, inflammatory and allergic diseases.
Investment Thesis:
GentiBio creates optimised Tregs phenotypes tailored to address a wide variety of autoimmune, alloimmune, inflammatory diseases and allergic diseases. Company’s proprietary platforms like DURA-REG (IL-2 signaling support), ENFUEL and Tissue Targeting are designed to create highly potent, stable, and scalable engineered Tregs with optimised safety and efficacy.
Lead Asset:
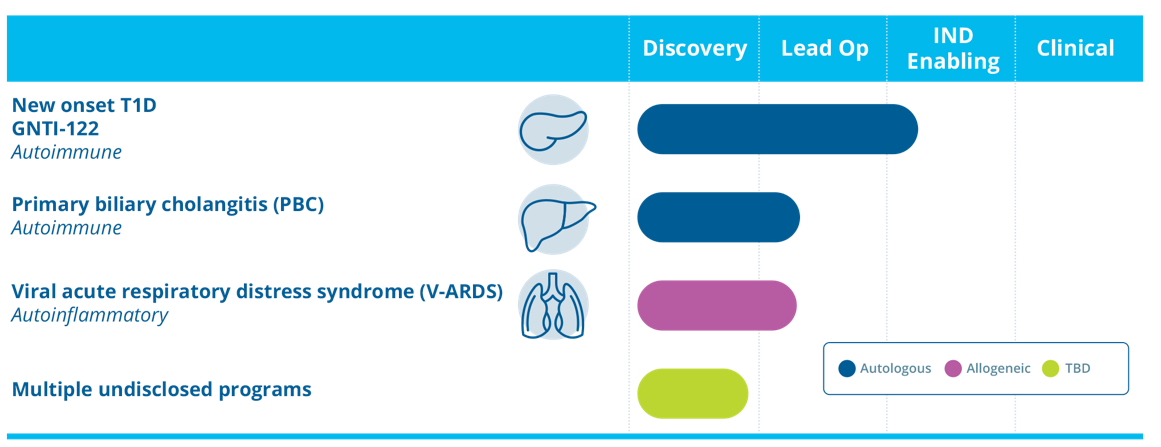
GNTI-122 is an autologous engineered Tregs product designed to protect beta cells from destruction by the immune system. GNTI-122 is designed to be a highly selective, potent, and durable immune modulatory therapy that is tunable after administration.
GNTI-122 is engineered to express a pancreatic islet-specific TCR to target the engineered Tregs to the site of disease together with modifications to stabilize and increase expression of the FOXP3 protein and the insertion of a chimeric IL-2 signaling complex, which provides exclusive proliferative and functional support.
Findings from preclinical study of GNTI-122 demonstrated stable Treg phenotype with homing of Tregs to islet cells and significantly reduced islet cell destruction and inflammation associated with the development of T1D.
The company also has a collaboration with Bristol Myers Squibb for the development of Treg therapies for IBD.
Unmet medical need:
T1D patients is a serious autoimmune disease caused by T-cell mediated destruction of insulin producing pancreatic beta cells. Patients need daily glucose monitoring and insulin administration. T1D is also associated with debilitating and life-threatening outcomes such as diabetic ketoacidosis, hypoglycemic events and micro- and macrovascular complications such as ischemic heart disease, strokes, retinopathy, and nephropathy. Current insulin replacement options are insufficient to replicate normal glycemic control, and for this reason, disease modifying therapies that can prevent or delay beta cell loss and maintain normal glucose control are urgently needed.
Market Opportunity:
An estimated 64,000 people are diagnosed with T1D each year. The disease is growing at a rate of 2.9 percent per year and by 2040, 2.1 million people are expected to be diagnosed with T1D.
Key Risks:
Children developing T1D have a more aggressive disease with less residual beta-cell function at presentation. Therefore, early intervention will be the option with Treg based treatments.
Financials:
GentiBio has raised a total of $177M in funding over 2 rounds. Their latest funding was raised on Aug 11, 2021 from a Series A round. GentiBio is funded by 7 investors. JDRF T1D Fund and Novartis Venture Fund are the most recent investors.
3.5 Abata Therapeutics (Private, raised a total of $95M)
Abata is based in Cambridge, Mass. Company was launched in 2021 by Third Rock Ventures with a $95 million Series A financing.
Investment Thesis
Developing engineered antigen-specific Treg cells for serious tissue-specific autoimmune diseases like progressive multiple sclerosis, T1D, and inclusion body myositis.
Lead Asset:
ABA-001: Lead program targets MS patients with non-relapsing progressive disease, imaging evidence of ongoing inflammatory tissue injury and an HLA genotype of DR2a/b. Tregs will be armed with a TCR that selectively recognizes degraded myelin.
A TCR engineered Treg will recognise degraded myelin and enable activation through antigen-dependent APC interaction. Thereafter, activated Tregs will release factors that suppress lymphoid aggregates, block new lymphocytes and inhibit inflammatory macrophages from degrading myelin. These engineered Tregs will also produce factors that may promote myelin repair.
Preclinical in vitro studies has demonstrated antigen-specific, dose-dependent Treg functionality, anti-inflammatory cytokine production, and suppression of the production of inflammatory factors (T cell-derived cytokines) of ABA-101.
Other preclinical products: T1D program for patients with the genetic HLA haplotype DR3-DQ2 (over half of patients) who are early in autoimmune pathogenesis, ultimately aiming to prevent the onset of symptomatic disease and insulin dependence; Inclusion Body Myositis: Treg cell therapy will treat patients with the genetic HLA haplotype DR3, an estimated 75% of patients.
Unmet medical need:
There are no approved therapies for non-relapsing progressive MS.
Market Opportunity:
Estimated 45,000 patients with non-relapsing progressive MS in the U.S. today.
Key Risks:
No clinical data to back the efficacy of product in humans.
Financials:
Abata Therapeutics has raised a total of $95M in Series A round on Jun 23, 2021. Company is funded by 6 investors with Samsara BioCapital and JDRF T1D Fund being the most recent investors.
3.6 AZTherapies (Private, raised a total of $130M)
AZTherapies is a private company headquartered in Boston, Massachusetts. It is an advanced clinical-stage biopharmaceutical company developing novel small molecules and biologic therapies that aim to fundamentally change neurodegenerative disease progression, extending normal cognition and function and improving quality of life in the aging population.
CAR-Treg Platform
Company’s CAR-Treg program is in pre-clinical stage, target antigen is undisclosed at the moment, that could have broad application across a spectrum of neurodegenerative diseases. Currently company is validating the efficacy of CAR-Treg platform in several models of neurodegenerative diseases.
AZTherapies are leveraging their proprietary technology to generate allogeneic, off-the-shelf cell therapies to reduce the patient burden and production hurdles associated with current therapies.
Financials
AZTherapies has raised a total of $130M in funding. Their latest funding raised $33.6M from a venture-Series C-1 Preferred Stock financing. The Series C-1 round was led by Malaysia-based publicly listed pharmaceutical company and new investor Duopharma Biotech Berhad. Company’s previously announced Series C Preferred Stock financing raised an aggregate gross proceeds of $37.5M. AZTherapies is funded by 5 investors. IBS Capital and Wexford Capital are the most recent investors.
3.7 Terraimmune (Private, raised $3M as Debt Financing)
TeraImmune, Inc. is a biotechnology company, based in Korea, founded in 2016 for development of innovative immune-cell therapies for curing autoimmune diseases.
Investment Thesis
TeraImmune is developing engineered Tregs from both natural Tregs isolated from patients and induced Tregs converted from a patient's T-effector (Teff) cells. They have proprietary Treg platform technology-
TREGable patented technology to generate large number of nTreg (CD4+CD25hiCD127lo/-Foxp3+Helios+) without losing their suppressive activity and
TREGing for production of an immunosuppressive iTregs (CD4+CD25+Foxp3+) by culturing isolated conventional T cells (CD4+CD25hiCD127lo/-Foxp3+Helios+) or naïve T cells (CD4+CD25-/loCD127+CD45RA+) for 2-3 weeks in culture.
With their patented technology, TeraImmune has resolved the technical hurdles to manufacture the FVIII-specific immune cell therapies for FVIII Anti-Drug Antibody in hemophilia A.
Lead Asset:
TI-168: next-generation, FVIII specific Treg therapy designed to reliably and effectively address Hemophilia A patients with FVIII inhibitor. Preclinical studies has successfully demonstrated the therapeutic potential of FVIII TCR-Treg therapy in controlling of FVIII Anti-Drug Antibody in a hemophilic animal model.
TeraImmune recently received FDA IND Clearance to initiate Phase 1/2a clinical Trial of TI-168 for treatment of Hemophilia A with Refractory Inhibitors.
The Company has expanded its pipeline to include the allogenic, or off-the-shelf, Tregs obtained from Umbilical Cord Blood for the treatment of the skin disease such as Atopic Dermatitis.
Company is also developing TCR-Treg therapies for MS and Xeno-transplantation, but, the specific targets are not disclosed.
Unmet medical need:
Hemophilia A is a rare genetic bleeding disorder that is caused by a lack of clotting factor VIII (FVIII). Standard care of treatment for these patients is FVIII replacement, but, the formation of anti-FVIII antibody is often problematic in the FVIII-replaced HA patients. The induction of FVIII Immune Tolerance is currently the only treatment option, which costs an average of $1M annually per patient. Thirty percent of the patients with FVIII Immune Tolerance still fail to control the FVIII Anti-Drug Antibody, rendering the treatment ineffective.
Market Opportunity:
There are between 30,000 – 33,000 males with hemophilia in the US. More than half of people diagnosed with hemophilia A have the severe form.
Key Risks:
Complex manufacturing, lack of human data to substantiate the potential of TCR-Treg therapy for HA.
Financials:
TeraImmune has raised a total of $3M in funding over 1 round. This was a Debt Financing round raised on Dec 22, 2022.
3.8 Kyverna Therapeutics (Private, raised a total of $110M)
Based in the San Francisco East Bay, Kyverna was Founded in 2018.
Financials: Kyverna Therapeutics has raised a total of $110M in funding over 2 rounds. Their latest funding was raised on Jan 26, 2022 from a Series B round. Series A investment from Vida Ventures, Westlake Village BioPartners, and Gilead, followed by a Series B financing in January 2022 led by Northpond Ventures.
4. Challenges of CAR-Treg therapy
Biggest challenge with the translational success of CAR-Treg therapy is the complex and costly manufacturing process of cell and gene therapies. The current manufacturing process for CAR-Tregs demonstrates several challenges in cell purification, yield, expansion, vector production/transduction, cryopreservation, and product release testing (see the below Flowchart ). Read this review in Cell: Toward an Optimized Process for Clinical Manufacturing of CAR-Treg Cell Therapy to understand the challenges of CAR-Treg therapy.

5. Conclusion
CAR-Treg cells are like “polypharmaceuticals”, they possess multiple therapeutic activities within a single medicine, that attack autoimmune and inflammatory diseases by interacting directly with the cells involved in the immune response and switching them off as well as indirectly targeting them by changing the metabolism at the site of inflammation, resulting in durable, lasting clinical response. Now clinical development of Treg based therapies is on the horizon and significant investment is being made by pharmaceutical companies. Further optimisations are required for the widespread use of engineered Tregs and to reduce the treatment cost, yet they remain a powerful and promising modality which may provide significant therapeutic benefit to numerous diseases reaching beyond transplantation and autoimmune diseases.
We are ushering in a new era of medicine, using Treg cells as living therapies to help restore and reset the immune system when it goes awry.