Dear Readers,
This article will explore three more innovator CAR-T companies: BLUE, TAK, XBIO! If you are new to SciX newsletter and want to get a view of what to expect in the articles, please click here.
Now let’s dig in
13. Bluebird Bio (Nasdaq: BLUE, M.Cap: US$ 1.24b)
Founded as Genetix Pharmaceuticals in April 1992 by MIT faculty members Philippe Leboulch and Irving London. In September 2010, the company was renamed Bluebird bio and Nick Leschly was named Chief Executive Officer. The company became public via an IPO in 2013. Company’s goal is to recode the science, the system–and even the status quo–for life. Company employs three gene therapy technologies: gene addition, cell therapy and (megaTAL-enabled) gene editing to find cure for spectrum of disorders including cerebral adrenoleukodystrophy, sickle cell disease, β-thalassemia and multiple myeloma. Here, we will only elaborate on the cell therapy portfolio of company.
Bluebird Bio and Bristol Myers Squibb got the most recent FDA approval for its CAR-T therapy for treatment of multiple myeloma. On March 26, the FDA approved Idecabtagene vicleucel (ide-cel, also called bb2121, ABECMA), a B-cell maturation antigen (BMCA)–directed CAR T-cell therapy for people with r/r multiple myeloma that has not responded to or has returned after at least four different prior cancer treatments. Approval for Abecma or ide-cel was based on data from the pivotal Phase II KarMMa study. This BMCA-CAR-T therapy showed 73% ORR, 33% CRR, mean duration of response in CR: 21.5 months. CAR T-cell toxic effects in r/r MM patients were expected, it was observed that almost all patients had grade 3 or 4 toxic effects, most commonly haematological toxic effects and cytokine release syndrome.
Design of Ide-cel: Autologous T cells were transduced with lentiviral vector encoding a second-generation CAR incorporating an anti-BCMA* single-chain variable fragment, a CD137 (4-1BB) costimulatory motif, and a CD3-zeta signaling domain. *BCMA is a potential target for multiple myeloma, it is primarily expressed by malignant and normal plasma cells and some mature B cells.
Ide-cel was granted Orphan Drug Status by both FDA and EMA. Also, it was granted Breakthrough Therapy designation by FDA and PRIME eligibility by EMA for r/r MM patients which helped in expediting its move from the lab to market. Bluebird and BMS has also received Orphan Drug status for their next-generation anti-BCMA CAR T cell therapy, bb21217 (CRB-402).
bb21217 uses the same scFv, 4-1BB costimulatory motif and CD3-zeta T cell activation domain as bb2121 with the addition of phosphoinositide 3 kinase inhibitor bb007 during ex vivo culture to enrich the drug product for T cells displaying a memory-like phenotype. This modification is done to increase the in vivo persistence of CAR-T cells and provide a cure for heavily treated r/r MM patients. Early clinical trial (NCT03274219) data appears to be promising, potential durable response following bb21217 CAR T cell treatment was observed, with a median duration of response of 11.1 months at the 150 x 106 CAR+ T cell dose level. Also durable CAR T cell persistence in evaluable patients (n=2/2) with ongoing response at up to 18 months following treatment was observed.
Besides, idecabtagene vicleucel they have two more products in the market:
Elivaldogene autotemcel (Skysona), a one-time gene therapy for the treatment of early cerebral adrenoleukodystrophy (CALD). CALD is a progressive and irreversible neurodegenerative disease that involves the breakdown of myelin, the protective sheath that nerve cells need to function effectively, especially for thinking and muscle control.
Betibeglogene autotemcel (Zynteglo), a medication for the treatment of the beta thalassemia group of inherited blood disorders. Zynteglo is manufactured using the BB305 a third-generation, self-inactivating lentiviral vector. The promoter for BB305 is a cellular non-viral promoter that drives gene expression only in the erythroid lineage cells.
The company plans to launch two independent, fully integrated commercial stage companies by the end of 2021: BLUE SGD (gene therapy for rare diseases) and 2seventy bio (oncology assets).
Advancements
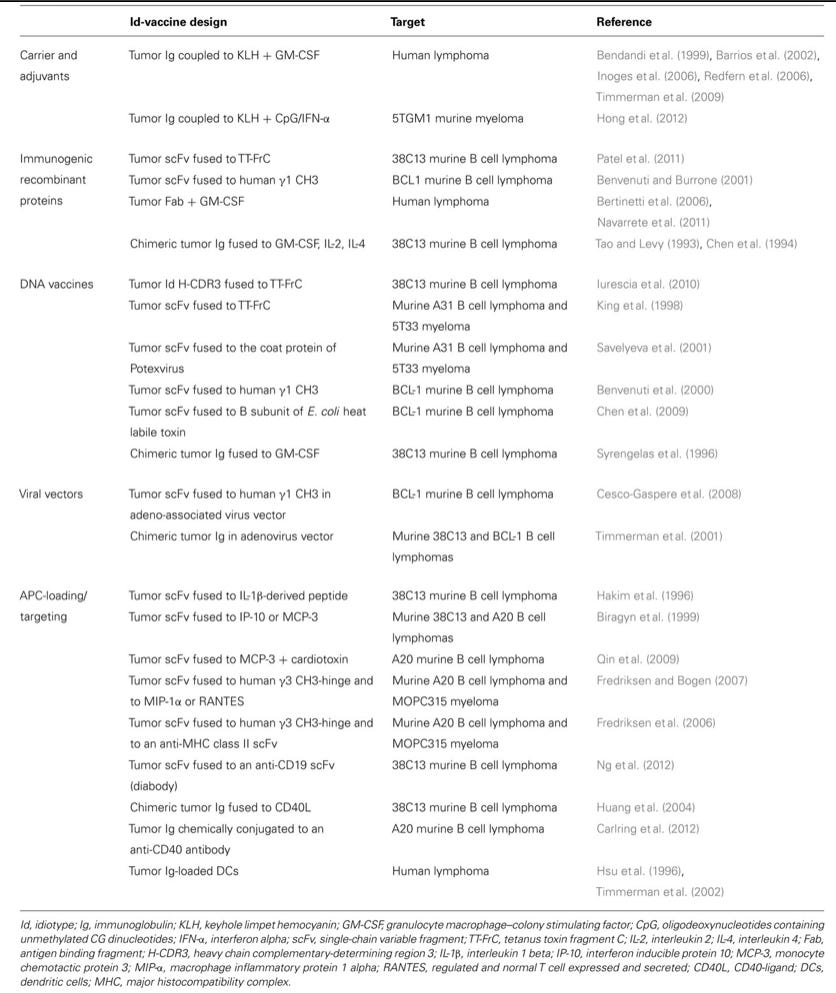
Multi-layered Enhancements to Deliver Improved Potency and Patient Outcomes: bbT369 CAR-T for bNHL patients. This CAR targets dual antigens, CD79a and CD20*; lacks Cbl proto-oncogene B (CBLB) gene which is negative regulator of T cell function (this strategy enhances potency and reduce exhaustion of T cells) and comprises two co-stimulatory domains, 41BB and CD28.
*CD20 and CD79a, have gained widespread acceptance because they are considered to be largely B-lineage restricted. The CD79a protein is present on the surface of B-cells throughout their life cycle, and is absent on all other healthy cells. CD20 is expressed on all stages of B cell development except on early pro-B cells or plasma blasts and plasma cells. In combination they are potential targets for bNHL patients or any B cell related neoplasms.

Source: Article, IntechOpen Pre-clinical data demonstrated that bbT369 CAR-T outcompetes anti-tumor activity of CD19 CAR-T in challenging low antigen models.
Dual targets with enhanced cellular signalling: NG-MM CAR-T which targets dual antigen, BMCA and other target which is undisclosed at present and also contains PI3K signalling enhancement
Regulatable CAR T (Drug-Regulated CAR-T cells or DARIC): CD33-CAR-T for AML with drug-regulation which enables drug-controlled ON/OFF states
Switch Receptor: Engineered TCR with enhanced potency (Superior MAGE-A4 TCR), Switch Receptor neutralizes TGFβ and activates IL12R signaling
VHH binders corresponds to the variable region of a heavy chain, versatile, very small size, humanised binders that can access challenging epitope spaces like VHH in DARIC CD33-CAR-T can access C2 domain of CD33 gene. This ensures target abundance across genotype and also prevents antigen escape.
If DARIC design proved to be safe and efficient in trials, company wants to improve the design further by multiplexing antigen targets (Tan-DARIC)
Product Pipeline
Recently Helixmith retrieved the rights for its CAR-T therapy VM801, targeting TAG-72 antigen from Bluebird Bio.
Their clinical manufacturing facility is under construction in its headquarters building, Cambridge, MA
Partners
BMS: collaborated to develop ide-cel (bb2121) and now another candidate, bb21217 is in development
Regeneron and Medigene: collaboration for development of MAGE-A4
Gritstone Oncology: to validate targets and discover TCR product candidates in the field of cancer.
Inhibrx: Collaborated in 2019 to use Inhibrx’s proprietary single domain antibody (sdAb) platform to multiple cancer targets.
TC BioPharm: In 2017, entered a strategic research collaboration and licensing agreement with TC BioPharm to discover and develop gamma delta CAR T cell product candidates for cancer immunotherapy.
Resilience will acquire bluebird’s Research Triangle (bRT) manufacturing facility located in North Carolina and Resilience will continue to support vector supply for both bluebird and 2seventy.
Academic collaborations at various stages or research and preclinical development at the Seattle Children's Research Institute, University of North Carolina, and the Fred Hutchinson Cancer Research Center.
Financials
Cash equivalents as of June 30, 2021 is US$ 941.6m (Net cash of US$641m) which provides a runway of 6-12 months at the current burn. Company expects US$ 110m upfront payment upon closing of the National Resilience strategic collaboration. At the end of 2021, company plans to separate into two public traded companies, bluebird and 2seventy. This quarter, bluebird and BMS launched BMCA-CAR-T for MM (ABECMA) in the US, which generated revenue of US$ 24m in 2Q of 2021 (shared 50% with BMS).
14. Takeda Pharmaceutical (NYSE: TAK, M.Cap: US$ 53.8b)
Takeda has a well structured Oncology Business Unit to meet the unique needs of the cancer community. They have several oncology drugs (ALUNBRIG, ICLUSIG, ADCETRIS, NINLARO, LEUPRORELIN AND VELCADE) in market around the globe (in more than 70 countries) with Global Oncology Revenue of US$ ~$3.9B for the FY2020.
Advancements
Cold-to-Hot Tumor: assets like TAK-981 and TAK-647 are developed to enhance the immune system’s recognition of cancer to drive immune activation and Tumor Regression. They achieve this by employing SUMOylation inhibitor (TAK-981) or via the use of STING (STimulator of INterferon Genes) agonists (TAK-647), both help in removing the brakes on endogenous IFN signalling and activating the immune system to attack cancer. Several Ph1/2 studies are ongoing.
T-cell engager COBRA Platform: Recently, Takeda advanced its immune-oncology portfolio by acquiring assets (TAK-186, TAK-280) developed by Maverick Therapeutics by using their COBRA platform. COBRA, which stands for COnditional Bispecific Redirected Activation, takes advantage of the tumor’s highly proteolytic microenvironment for T-cell activation. T cell engagers are differentiated T cell engagers with extended half-life designed to be conditionally activated in solid tumor microenvironment.
TAK-186 (formerly MVC-101) targets EGFR-expressing solid tumors such as those found in head and neck cancers and TAK-280 (formely MVC-280) targets B7H3-expressing solid tumors such as prostate cancer.
Ongoing Phase 0 master protocol using the CIVO® platform (collaboration with Presage Biosciences) to evaluate intratumoural microdoses of anti-cancer therapies (TAK-186 alone or in combination with Nivolumab) in patients with solid tumours (NCT04891718) also currently in a Phase 1/2 study to evaluate the safety, tolerability, pharmacokinetics, pharmacodynamics and preliminary antitumor activity of MVC-101, an EGFR x CD3 COnditional Bispecific Redirected Activation (COBRA™) Protein in patients with unresectable locally advanced or metastatic cancer (NCT04844073).
Allogenic CAR-NK cells (iC9/CAR.19/IL15-Transduced CB-NK Cells): TAK-007, Cryopreserved IL-15 armored cord blood-derived CD19-targeted CAR-NK therapy
Clinical trial to evaluate the safety and tolerability in adult participants with r/r B-cell NHL is ongoing (NCT05020015)
Early Data from 11 patients with non-Hodgkin’s lymphoma and chronic lymphocytic leukemia showed 8 responding to the therapy, including 7 complete response. After a median of 13.8 months’ follow-up, patients continued to show no evidence of disease, and 5 received post-remission therapy. Importantly, none of the patients experienced the toxicities associated with CAR-Ts, such as neurotoxicity and cytokine release syndrome (CRS).
We will discuss in detail about the biology of NK cells and CAR-NK cells in upcoming articles, stay tuned!
Product Pipeline
Company’s CAR assets under Redirected Immunity
They have cGMP manufacturing facility at R&D HQ, Cambridge (USA) and commercial manufacturing facility with versatile design in Lexington (USA).
Partners
Presage Biosciences: collaboration for their CIVO (Comparative In Vivo Oncology) platform, with this company can perform Phase 0 microdose trials to evaluate the localized tumor response to drug candidates in the tumor microenvironment while also capturing tumor heterogeneity and patient diversity.
Crescendo Biologics: Takeda has exercised an option from Crescendo Biologics for an exclusive oncology-targeted Humabody license, allowing it to evaluate Humabody VHs for the development of novel CAR-T therapeutics.
Noile-Immune Biotech: secured exclusive license rights to NIB-102 (GPC3 CAR-T) and NIB-103 for the treatment of solid tumor indications
Memorial Sloan Kettering Cancer Center: collaborated to develop novel CAR-T products for the treatment of multiple myeloma, acute myeloid leukemia and other solid tumors.
Key management
Teresa Bitetti: President, Global Oncology Business Unit and Chris Arendt: Head, Oncology Therapeutic Area Unit
Financials
For Q1 FY2021 ended June 30, 2021, Takeda’s reported revenue increased +18.4% to US$ 8.6b, where Oncology platform contributed to US$ 1.1b. Free cash flow generated in the latest quarter was US$1.18b.
15. Xenetic Biosciences (NASDAQ: XBIO, M.Cap US$23.8m)
The company was founded in 1997 and listed on NASDAQ via IPO in 2016. It is a biopharmaceutical company focused on advancing XCART, a personalized CAR T platform technology engineered to target patient- and tumor-specific neoantigens. Additionally, the company has developed advances in drug delivery platform with PolyXen.
Advancements
XCART Platform: a personalised CAR T platform technology engineered to target patient-specific tumor neoantigens that target independently of CD19 or other surface antigens that are common to both normal and malignant B-cells. This technology was originally developed by Scripps Research (independent, nonprofit biomedical institute) in collaboration with the Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry (the largest center of physical and chemical biology and biotechnology in Russia).
While currently approved therapies for B-Cell Non-Hodgkin's lymphoma are designed to target CD19, a B cells receptor, which is expressed on both malignant and normal B cells, XCART is designed to target patient-specific, tumor-specific antigens that are independent of CD19 or other antigens common to all B-Cells.
The XCART technology platform utilises an established screening technique to identify polypeptide domains that selectively bind to the unique B-cell receptor* on the surface of an individual lymphoma patient’s malignant B-cell clones. This BCR-selective targeting domain is engineered into the antigen-binding domain of a CAR, creating the possibility of a CAR T treatment that should only recognise a given patient’s malignant B-cell clones.
*Non-Hodgkin B-cell lymphomas are almost invariably derived from B lymphocytes that have undergone productive V(D)J rearrangements of their immunoglobulin genes and may additionally have experienced somatic hypermutation. The resulting B-cell receptor idiotype contains novel, tumor-specific sequences and thus represents a class of neoantigen unique to B-cell malignancies. The individual composition of potentially immunogenic epitopes of any immunoglobulin is designated as “idiotype.” (Blood, 2019)

Despite bearing a unique gene combination, and thus particular epitopes, several studies have shown that it is normally difficult to stimulate the immune response against antibody variable regions. Click here if interested to read further on strategies of boosting immune response. Company might have thought about this as understood from their patent application which covers co-administration of XCART-derived CAR T cells together with a personalized vaccine designed to enhance the effectiveness of the CAR T therapy.
This approach will limit off-tumor toxicities, such as B-cell aplasia and also limit the relapse of CD19 negative B cell lymphoma. Current data shows that approx. 50% patients recur within 12 months after CD19-directed CAR-T therapy.
Biopsy and blood samples from B-Cell NHL patients are used for isolation and screening of tumor-specific neoantigens, and potential tumor specific CARs are designed and characterised to evaluate XCART platform.
On Sep 9, 2021 the United States Patent and Trademark Office (USPTO) issued the company a Notice of Allowance for U.S. patent application number 16/983,491 entitled, "Articles and Methods Directed to Personalized Therapy of Cancer”. The U.S. patent scheduled to issue from this application will expire in 2038.
PolyXen platform (A Polysialylation Technology): proprietary drug delivery platform for next-generation protein drug delivery. The natural polymer polysialic acid (linear alpha-2-8 polymer of sialic acids) is employed to modulate the pharmacokinetic and pharmacodynamic properties of protein drugs
PolyXen has demonstrated its ability to improve the half-life and other pharmacological properties of next-generation biologic drugs. Some of the features are listed below:
The Company has an exclusive license agreement with Takeda Pharmaceuticals Co. Ltd. in the field of coagulation disorders and receives royalty payments under this agreement.
Product Pipeline
Partners
Scripps Research: collaborated to advance XCART technology, they are one of the original developers of this technology. Scripps Research is an independent, nonprofit biomedical institute ranked the most influential in the world for its impact on innovation.
On June, 2020 the company entered into a master services agreement with Pharmsynthez and several academic institutes* to advance the development of the XCART technology for B-cell malignancies. Pharmsynthez (MOEX: LIFE) is a Russian pharmaceutical company that develops new medicines, drug technologies for organ-specific delivery, and innovative methods of manufacturing pharmaceutical ingredients.
*Academic collaborations: 1. Shemyakin and Ovchinnikov Institute of Bioorganic Chemistry of the Russian Academy of Sciences (IBCh RAS); 2. Belarussian Research Center for Pediatric Oncology, Hematology and Immunology; 3. Institute of Bioorganic Chemistry of the National Academy of Sciences of Belarus; 4. Vitebsk Regional Clinical Oncological Center
Shire: collaborated for PSA for Factor VIII (hemophilia)
Serum Institute of India: Manufactures cGMP grade ErepoXenTM (PSA-EPO)
Key management
Jeffrey F. Eisenberg: Chief Executive Officer & Director
Financials
Cash balance was US$ 9.3m as of June 30, 2021 which should provide runway for 12 months odd.