Dear Readers,
Welcome to the first article of SciX newsletter for Biocurious enthusiasts-whether from the industry or academia or the financial world!
In the last few decades, cancer treatments have seen a paradigm shift from broad pan treatments (chemotherapy, radiation, hormonal therapy) to personalised therapy. This became possible with the emergence of immuno-oncology drugs, which include a broad range of agents, including antibodies, vaccines, adjuvant therapies, cytokines, oncolytic viruses, bisepecific molecules, and cellular therapies.
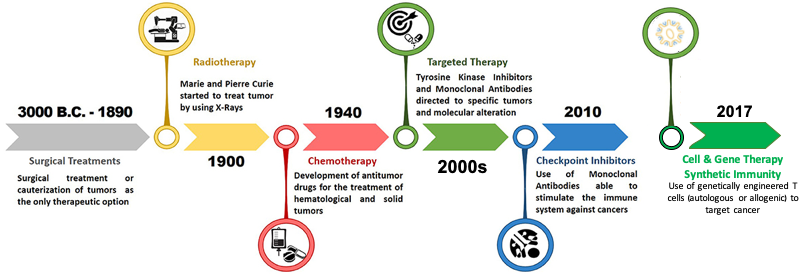
Chronologically, Adoptive T cell therapy (ACT):
At first it started with employing Tumor Infiltrating Lymphocytes (TILs) for treatment of melanoma. Limited access to resectable metastases or tumors, time-consuming T cell preparation, and scarce tumor-reactive T cell clones have so far hindered this strategy’s success.
Secondly, T cells genetically engineered with T cell receptor (TCR) started being generated to tackle some major pitfalls of TIL therapy. This specificity remains inherently restricted because of its dependence on antigens expressed by tumors via their MHC complexes.
The third ACT approach to reach the spotlight consists of the so-called chimeric antigen receptor (CAR) modified T cells, gaining an edge over the previous two with an ingenious series of modifications.
In this article, we will unravel the world of breakthrough cellular therapy—CAR-T, which employs T-cell engineering and synthetic biology approaches. All through, the focus of this article series will be first to unravel the scientific world of CAR-T in haematological cancers (Part-1), solid tumors (Part-2) and thereafter understand the fast-growing and evolving CAR-T market, translational world of CAR-T (Part-3 and Part-4).

CAR-T constitutes a powerful new class of therapeutic agents, it employs the body’s “serial killer” cells (T cells) as an elixir to fight cancer. Henceforth, also referred to as “living therapy”.
Advances in T-cell engineering, genetic editing, the selection of the most functional lymphocytes, and cell manufacturing have the potential to broaden T-cell–based therapies and foster new applications beyond oncology in infectious diseases, organ transplantation, and autoimmunity. The emerging market of CAR-T beyond oncology will be delved in detail in future articles, stay tuned!
1.1 Background on CAR Therapy
CARs do not exist in nature, they are engineered receptors. Since this designed concoction is part antibody and part T-cell, it is a chimera, like the monster of Greek mythology that is part lion, part goat and part serpent. It has synthetic immune receptors that are able to confer antigen-binding and activating functions on T cells with the aim of therapeutically targeting cancer cells. So the whole construct is called a chimeric antigen receptor, or CAR, and the use of it to treat cancer is called CAR T-cell therapy, or CAR-T.
In 1987, an immunologist, Dr. Zelig Eshhar from The Weizmann Institute of Science, paved the way for a chimeric cancer therapy. Dr. Steven Rosenberg of U.S. National Cancer Institute was the first to dare to make use of this CAR therapy in clinical experiments. Other pioneers of CAR-T therapy are Dr. Carl H. June of the University of Pennsylvania and Dr. Michel Sadelain of Memorial Sloan Kettering Cancer Center. The latter three scientists labouring in separate labs have doggedly pursued a dream — turbocharging the body’s immune system to fight cancer, a daring therapy that few colleagues believed would work. Another early developer of CAR-T was Dr. Dario Campana of St. Jude Children’s Research Hospital. He along with Dr. Chihaya Imai first published the CAR design in Leukemia in 2004.
It’s ironic that the mutations that caused the cancer may turn out to be the cancer’s Achilles’ heel and enable successful treatments-Steven A. Rosenberg

In the 1990s and early 2000s, scientists worked tirelessly perfecting the formula. Success story of the therapy was first published by Dr. Rosenberg and colleagues, in the journal Blood in 2010. However, it was Dr. June’s publication of 2011 that transformed the field. The success story of complete remission of Chronic Lymphoid Leukemia (CML) patients drew attention of Novartis, the big Swiss pharmaceutical company (NYSE: NVS). In 2012, Novartis licensed the exclusive worldwide license to the CAR-T cell technology developed in Penn. That set off a commercial rush, flooding the field with cash after years of doubt. Additionally, this alliance also expanded the 2012 lawsuit by Juno Therapeutics (Nasdaq: JUNO) as they had licensed CAR technology from St. Jude Children’s Research Hospital. Why the lawsuit? Because, the actual DNA used to design CAR-T by Dr June was “designed, developed, and provided” by Dario Campana and Chihaya Imai, who worked at St. Jude Children’s Research Hospital. The legal case settled in 2015 with Novartis paying $12.5 million and shifting some future payments from Penn to St. Jude as well as to a startup named Juno Therapeutics. In 2018, this company was acquired by Celgene (Nasdaq:CELG), which in the next year was acquired by Bristol-Myers Squibb (NYSE:BMY).
In 2012, Rosenberg’s group core technology of generating engineered autologous T cell therapy (eACT) was bought by Kite Pharma (Nasdaq: KITE), a pharmaceutical company founded by Rosenberg’s former NCI trainee Arie Belldegrun. In 2017, Kite Pharma was acquired by Gilead Sciences (Nasdaq: GILD).
1.2 Arming T cells to target and destroy cancer cells
1.2.1 How are CAR-T cells created ?
As its name implies, the backbone of CAR T-cell therapy is T cells, which are often called the workhorses of the immune system because of their critical role in orchestrating the immune response and killing cells infected by pathogens. The therapy requires drawing blood from patients and separating out the T cells. Next, using a disarmed virus (like, lentiviral and retroviral vectors), the T cells are genetically engineered to produce receptors on their surface called chimeric antigen receptors, or CARs. If interested, read the review on Global Manufacturing of CAR-T cell therapy.
Recent advances in gene editing technology (like CRISPR/Cas9) and oncology research have now provided tools to bio-engineer advanced CARs and also reduce the time of manufacturing to less than 7 days.
Let’s briefly discuss the structure of CAR T cell and it’s developmental evolution.
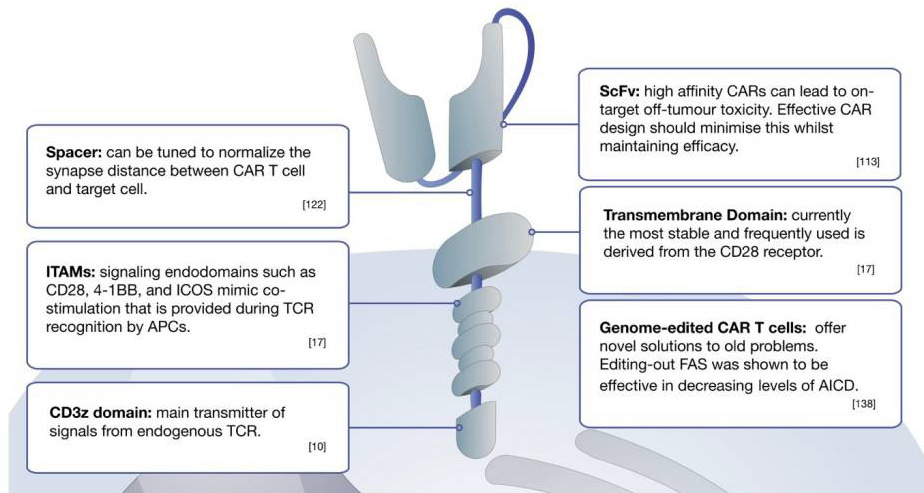
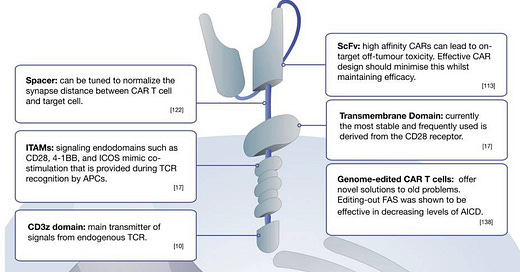
CARs comprise three main components
Extracellular domain: single-chain variable fragment (scFv) domain responsible for antigen (on tumour cells) recognition; unlike innate T cell receptors (TCRs), CAR-T can recognise antigens in the absence of presentation by the major histocompatibility complex (MHC)*
Transmembrane domain: fundamental for surface expression and stability of the receptor
Intracellular signalling domain (Endodomain): Following antigen binding, the intracellular domain clusters undergoes conformational changes, which enables downstream signalling proteins to be recruited and phosphorylated.
*Xplained: How conventional effector T cells, recognize antigens via MHC presentation
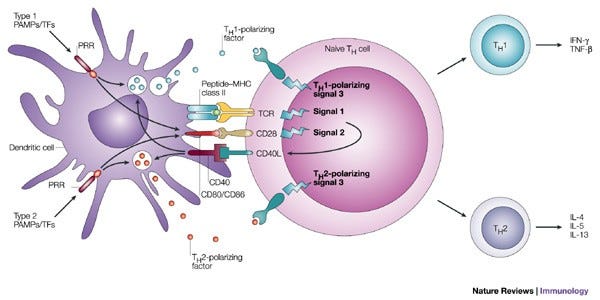
Antigen presenting cells like dendritic cells (DCs) or Macrophages (big-eaters) engulf pathogens and internally process them, the antigenic fractions of the pathogen are then flagged with MHC on the surface of these cells. Nïave T cells recognise these antigens through their innate TCRs, which stimulates and matures T cells into different effector T cells: a. T helper cells (CD4 T) which can differentiate into different subsets which intern activated different immune cells (i.e., Th1 cells activate macrophages and Th2 cells activate B cells); 2. T cytotoxic cells (CD8 T) that can lyse virally-infected or tumor cells.
1.2.2 Different CAR Models:
Based on the structure and composition of the endodomain, CAR can be roughly grouped into five generations:
First generation CAR was generated 30 years ago. It had only single CD3ζ (read as CD3zeta) intracellular domain. They showed low toxicity and could not promote CAR T cell proliferation.
Second Gen (currently approved therapies): Additional co-stimulatory domain, sections of CD28 or CD137 was incorporated into the intracellular signalling domain, which in turn augmented CAR-T’s potential to grow, expand in patient body
Third Gen (Developmental phase): additional co-stimulatory domain such as CD134 or CD137 on second Gen CAR.
Fourth Gen (Developmental phase): T cells redirected for universal cytokine-mediated killing (TRUCKs): The fourth generation of CARs is based on second-generation CARs, but include a protein, such as interleukin 12 (IL-12) that is constitutively or inducibly expressed upon CAR activation. Promote tumour killing though several synergistic mechanisms such as exocytosis (perforin, granzyme) or death ligand–death receptor (Fas–FasL, TRAIL) systems
Fifth Gen (Developmental phase): based on the second generation of CARs, but they contain a truncated cytoplasmic IL-2 receptor β-chain domain with a binding site for the transcription factor STAT3. The antigen-specific activation of this receptor effectively provides all three synergistic signals required physiologically to drive full T cell activation and proliferation.
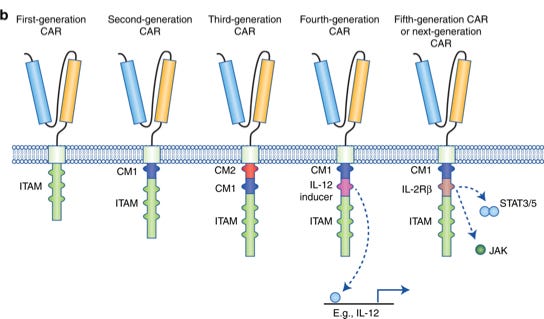
Currently it is unclear which design would provide the best clinical benefit for patient outcome.
1.2.3 CAR-T cells as potent “serial-killers”
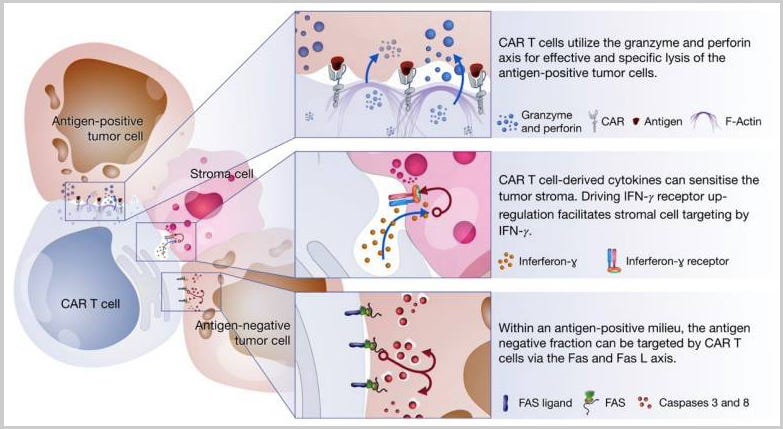
CAR T cells mediate tumor killing via three axes:
Perforin and granzyme axis: Targeting antigen positive fraction
Cytokine secretion: Stromal cell sensitization
Fas and FasL axis: Targeting antigen-negative fraction
1.3 FDA Approved CAR-T products
Time line of the CAR-T cell therapy which are already available on the market:
2017: marked the real advent of the CAR T era when Tisagenlecleucel (anti-CD19 with 4-1BB co-stimulation, sold as Kymriah), Novartis got US Food and Drug Administration (FDA) approval for the treatment of relapsed or refractory (r/r) B-cell precursor acute lymphoblastic leukemia (ALL). Clinical trials were done in collaboration with Carl June’s, team in Penn's Perelman School of Medicine, who is a pioneer of this new treatment. Clinical Trial identifier: NCT02435849; Six month relapse free rate of 80% was observed.
2018: Axicabtagene ciloleucel (anti-CD19 with CD28 co-stimulation, sold as Yescarta), got FDA approval for r/r DLBCL. Clinical Trial identifier: NCT02348216, ZUMA-1; Objective response was observed in 83% and 58% showed complete response.
2020: Brexucabtagene autoleucel (anti-CD19 with CD28 co-stimulation, sold as Tecartus), Kite Pharma (now under Gilead) got accelerated FDA approval for r/r Mantle Cell Lymphoma. Clinical Trial identifier: NCT02601313, ZUMA-2; Objective response was observed in 93% and 67% showed complete response.
2021: Lisocabtagene maraleucel (anti-CD19 with 4-1BB co-stimulation, sold as Breyanzi), Juno Therapeutics (now BMS) got approval for refractory B cell lymphomas. Clinical Trial identifier: NCT02030834; Objective response was observed in 64% and 43% showed complete remission.
Early CAR-T development, focussed on B-cell malignancies which expresses CD19*. CD19 is a suitable target for CAR T cells because it is expressed by B-cell malignancies, but not by normal essential tissues
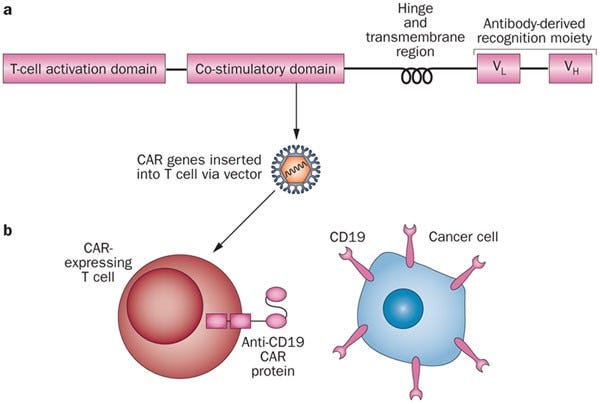
Design of CAR-T for B cell malignancies. Adapted from Nature Reviews Each CAR T cell has signaling and "co-stimulatory" domains inside the cell that signal the cell from the surface receptor. These different domains affect cells overall function
Depleting endogenous lymphocytes by administering chemotherapy or radiotherapy before infusions of adoptively transferred T cells enhances the in vivo activity of the T cells
Depletion of normal CD19+ B cells is associated with B-cell aplasia and hypogammaglobulinemia. These on-target, off-tumor toxicities may result in an increased risk for infection, particularly for encapsulated bacteria. Patient remains on lifelong prophylactic IgG replacement to prevent infections.
1.4 Complications with CAR-T therapy
Unparalleled clinical efficacy has been demonstrated using anti-CD19-CAR T cells to treat refractory CD19+ B cell malignancies. However, there are some complications with CAR-T, let’s go through this:
Cytokine Release Syndrome (CRS): As part of their immune-related duties, T cells release cytokines, chemical messengers that help to stimulate and direct the immune response. In the case of CRS, there is a rapid and massive release of cytokines into the bloodstream, which can lead to dangerously high fevers and precipitous drops in blood pressure. CRS issues can increasingly be tamed and are not a deal breaker.
CRES (CAR T-cell-related encephalopathy syndrome) and Neurotoxicity: another serious and potentially fatal side effect—swelling in the brain, or cerebral edema. Other so-called neurotoxicities—such as confusion or seizure-like activity—have been seen in most CAR T-cell therapy trials. But in nearly all patients the problem is short lived and reversible.
Resistance to CAR-T therapy: ability of tumors to hide from CD19-targeted CAR T cell therapy (epitope masking) represents a major threat to treated patients. Approaches which targets additional B cell antigens, such as CD22, to reduce the likelihood of tumor escape will be beneficial. Multiple studies reveal that patients do not respond to CD19/CAR-T if their cancer cells harbor mutated versions of the CD19 protein. Furthermore, exon skipping events can modify CD19 protein so that cancer cells remain undetected by CAR-T.
Huge Costs
Production time: Advanced techniques have reduced the time of production for autologus CAR-T cells from several weeks to 7 days now. Efforts are on to further reduce the time by designing allogenic CAR-T (off-the-shelf).Not only will they be faster to produce but also cheaper.
Next article of CAR-T Series will cover the challenges and opportunities of CAR-T in Solid Tumors and an in-depth discussion on Next Gen CARs…stay tuned!!



Very well written, thanks for sharing.